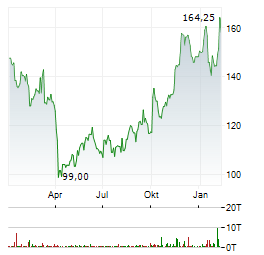
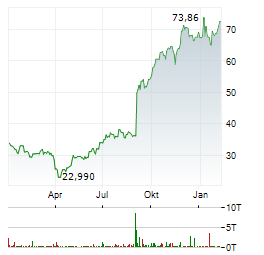
Chart-Typ:
Zeitraum:
| Johnson & Johnson Says AKEEGA Wins CHMP Backing For Metastatic Prostate Cancer With BRCA1/2 Mutation | NEW BRUNSWICK (dpa-AFX) - Johnson & Johnson (JNJ) announced Friday that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has recommended an indication... ► Artikel lesen |
| J&J Secures FDA Approval For DARZALEX FASPRO Combo In Newly Diagnosed Multiple Myeloma | NEW BRUNSWICK (dpa-AFX) - Drug major Johnson & Johnson (JNJ) announced Tuesday that DARZALEX FASPRO (daratumumab and hyaluronidase-fihj) -based quadruplet regimen has received the U.S. Food... ► Artikel lesen |
| Johnson & Johnson: DARZALEX FASPRO-based quadruplet regimen approved in the U.S. for newly diagnosed patients with multiple myeloma who are transplant ineligible | Quadruplet regimen demonstrated significantly deeper and more durable responses, higher MRD negativity and improved progression-free survival versus a standard of... ► Artikel lesen |
| Biogen Q4 Results Top Estimates; Guides FY26 Adj. EPS Above Estimates | WESTON (dpa-AFX) - Biotechnology company Biogen, Inc. (BIIB) reported Friday a net loss attributable to the company for the fourth quarter of $48.9 million or $0.33 per share, compared to net... ► Artikel lesen |
| Ihre wichtigsten Termine: Frische Zahlen von Philip Morris, Under Armour, Toyota, Biogen, Societe Generale | © Foto: Hiro Komae/AP/dpaGut geplant in den Tag. Mit dem wO-Tagesausblick haben Sie die wichtigsten Termine im Blick und starten bestens vorbereitet in den neuen Handelstag.Unternehmenstermine 06:30... ► Artikel lesen |
| Biogen: Phase 2/3 DEVOTE Study Supports Clinical Benefits Of High-dose Regimen Of nusinersen | WESTON (dpa-AFX) - Biogen (BIIB) announced that Nature Medicine published results from the Phase 2/3
DEVOTE study evaluating the high-dose regimen of nusinersen, comprised of 50 mg/5 mL loading... ► Artikel lesen |
| EU Approves DAWNZERA For Hereditary Angioedema, Says Ionis And Otsuka | TOKYO (dpa-AFX) - Ionis Pharmaceuticals, Inc. (IONS) and Otsuka Pharmaceutical Co., Ltd. on Wednesday said the European Commission has approved Dawnzera to prevent repeated attacks of hereditary... ► Artikel lesen |
| Ionis Pharmaceuticals, Inc.: DAWNZERA (donidalorsen) approved in the European Union for hereditary angioedema (HAE) | Ionis Pharmaceuticals, Inc. (Nasdaq: IONS) and Otsuka Pharmaceutical Co., Ltd. (Otsuka) today announced that the European Commission (EC) has approved DAWNZERA (donidalorsen) in the European Union... ► Artikel lesen |
| Ionis Pharmaceuticals, Inc.: TRYNGOLZA (olezarsen) approved in the European Union for familial chylomicronemia syndrome (FCS) | Ionis Pharmaceuticals, Inc. (Nasdaq: IONS) and Sobi® today announced that TRYNGOLZA® (olezarsen) has been approved in the European Union (EU) as an adjunct to diet in adult patients for the treatment... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu BG MEDICINE INC finden Sie auf Wallstreet Online.