| Realtime | Geld | Brief | Zeit |
|---|---|---|---|
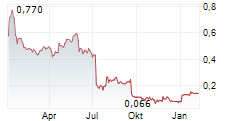
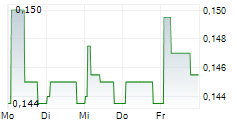
| 0,466 | 0,480 | 23:01 |
Chart-Typ:
Zeitraum:
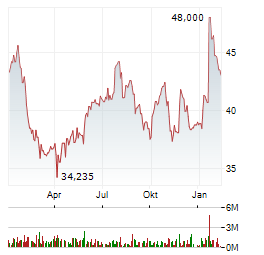
| Qiagen NV-Aktie heute am Aktienmarkt kaum gefragt: Kurs fällt (35,815 €) | Am Aktienmarkt notiert die Aktie von Qiagen NV aktuell etwas leichter. Das Wertpapier notiert derzeit bei 35,82 Euro. Ein Minus von 3,11 Prozent steht gegenwärtig für das Wertpapier von Qiagen NV zu... ► Artikel lesen |
| PTA-NVR: QIAGEN N.V.: Veröffentlichung der Gesamtzahl der Stimmrechte nach § 41 WpHG | DJ PTA-NVR: QIAGEN N.V.: Veröffentlichung der Gesamtzahl der Stimmrechte nach § 41 WpHG
Gesamtstimmrechte gemäß -- 41 WpHG
QIAGEN N.V.: Veröffentlichung der Gesamtzahl der Stimmrechte nach... ► Artikel lesen |
| AKTIEN IM FOKUS: Pharmawerte unter Druck - Zölle treffen Sektor hart | FRANKFURT (dpa-AFX) - Die eigentlich als defensiv geltenden Pharmawerte sind im frühen Handel am Mittwoch mit deutlichen Abgaben aufgefallen. Der Branchenindex Stoxx Europe 600 Health Care ist in einem... ► Artikel lesen |
| Tempus AI Aktie: Anstehende Entwicklungen im Check | Die Tempus AI Aktie steht aktuell im Fokus der Anleger, nachdem mehrere Direktoren des Unternehmens in erheblichem Umfang Anteile veräußert haben. Am 17. März 2025 haben drei Vorstandsmitglieder Aktien... ► Artikel lesen |
| Wieder zugeschlagen: Cathie Wood setzt auf Tempus AI - So will sie vom KI-Boom profitieren! | © Foto: ARK Invest/ Reed YoungNach einem Kursrutsch greift Cathie Wood massiv zu Tempus AI und setzt auf eine starke Erholung - die Aktie legt bereits 4 Prozent zu.Die bekannte Investorin Cathie Wood... ► Artikel lesen |
| wO Börsenlounge: Palantir | Hims | Tempus AI - DeepSeek kurbelt Chipnachfrage bei Nvidia an! | Tag der Sorgenkinder in der wO Börsenlounge. Die Aktie von Palantir fällt seit Tagen, Tempus AI bricht nach den Zahlen zweistellig ein und Hims & Hers steht trotz guter Zahlen kräftig unter Druck. Wie... ► Artikel lesen |
| SpringWorks Therapeutics, Inc.: SpringWorks Therapeutics Reports Fourth Quarter and Full Year 2024 Financial Results and Highlights Recent Business Updates | - Achieved $61.5 million and $172.0 million in fourth quarter and full year 2024 OGSIVEO® (nirogacestat) U.S. net product revenues, respectively - - Received FDA approval of GOMEKLI (mirdametinib)... ► Artikel lesen |
| Merck KGaA: Wechsel im Top-Management - wann schlägt der DAX-Konzern bei SpringWorks zu? | Die Aktie von Merck KGaA notiert weiterhin in der Nähe des 52-Wochen-Tiefs. Das bestätigte Übernahmeinteresse an SprinWorks Therapeutics hat den DAX-Wert jüngst unter Druck gesetzt, denn die Marktteilnehmer... ► Artikel lesen |
| SpringWorks Therapeutics: FDA hat es eilig - wann schlägt Merck KGaA endgültig zu? | Nach aufgekommenen Übernahmegerüchten rund um die Biotech-Gesellschaft SpringWorks Therapeutics durch einen Bericht der Nachrichtenagentur Reuters vonseiten der in Darmstadt ansässigen Merck KGaA herrscht... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und .
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu INTEGRAGEN finden Sie auf Wallstreet Online.