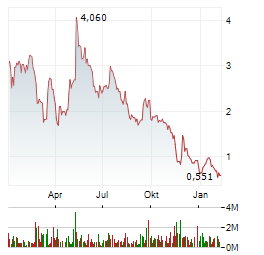
Chart-Typ:
Zeitraum:
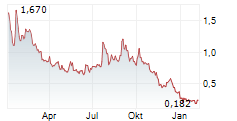
| 2026 - Das Comeback der Wasserstoffaktien: Jetzt zählt Substanz statt Story! Die versteckten Potenziale von dynaCERT, Ballard Power und VW | Jahrelang galten Wasserstoffaktien als Zukunftsversprechen. Dem großen Hype folgte die Katerstimmung. Die Bewertungen sind massiv gefallen, nach der Phase überzogener Erwartungen rücken nun belastbare... ► Artikel lesen |
| Ballard, Bloom Energy und Plug: Lohnt sich jetzt der Einstieg? | An den vergangenen Handelstagen ging es mit den Aktienkursen von Wasserstoff-Playern wie Bloom Energy, Ballard Power oder auch Plug Power und somit auch mit dem Wasserstoff Nordamerika Index des AKTIONÄR... ► Artikel lesen |
| Ballard Power: Umsatz dank Nordamerika mehr als verdoppelt - Verlust reduziert, bleibt aber Belastung | VANCOUVER, Kanada (IT-Times) - Der kanadische Brennstoffzellen-Entwickler Ballard Power hat seine Ergebnisse für das dritte Quartal 2025 veröffentlicht und ein dreistelliges Umsatzwachstum verzeichnet.... ► Artikel lesen |
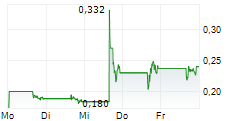
| EQS-News: The NAGA Group AG: The NAGA Group veröffentlicht am 12. Februar 2026 die vorläufigen Ergebnisse für das Geschäftsjahr 2025 - Einladung zum Earnings Webcast/Call am selben Tag | EQS-News: The NAGA Group AG
/ Schlagwort(e): Jahresergebnis/Sonstiges
The NAGA Group veröffentlicht am 12. Februar 2026 die vorläufigen Ergebnisse für das Geschäftsjahr 2025 -... ► Artikel lesen |
| THE NAGA GROUP AG: Earnings Call für Investoren am 12.02.2026: NuWays veranstaltet am 12.02.2026 um ... | Die NuWays AG veranstaltet einen Earnings Call mit der THE NAGA GROUP AG. The NAGA Group präsentiert seine vorläufigen FY 2025 Zahlen inklusiver einer Q&A Session mit dem CEO und CFO. Unternehmen: THE... ► Artikel lesen |
| Original-Research: THE NAGA GROUP AG (von NuWays AG): BUY | Original-Research: THE NAGA GROUP AG - from NuWays AG
19.12.2025 / 09:31 CET/CEST
Dissemination of a Research, transmitted by EQS News - a service of EQS Group.
The issuer is solely responsible... ► Artikel lesen |
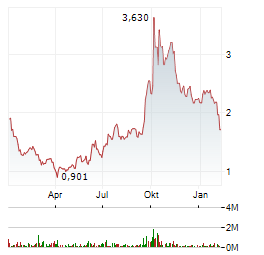
| EQS-News: DeFi Technologies Inc.: DeFi Technologies gibt bekannt, dass das Venture-Portfoliounternehmen Stablecorp die VersaBank als Verwahrstelle für den QCAD Digital Trust ausgewählt hat, wodurch Kanadas erste konforme ... | EQS-News: DeFi Technologies Inc.
/ Schlagwort(e): Vertrag
DeFi Technologies gibt bekannt, dass das Venture-Portfoliounternehmen Stablecorp die VersaBank als Verwahrstelle für... ► Artikel lesen |
| EQS-News: DeFi Technologies Inc.: DeFi Technologies gibt bekannt, dass Valour die behördliche Genehmigung in Großbritannien erhalten hat und nun ausgewählte renditestarke Krypto-ETPs über die Londoner Börse für britische Privatanleger anbietet | EQS-News: DeFi Technologies Inc.
/ Schlagwort(e): Vertrag/Vereinbarung
DeFi Technologies gibt bekannt, dass Valour die behördliche Genehmigung in Großbritannien... ► Artikel lesen |
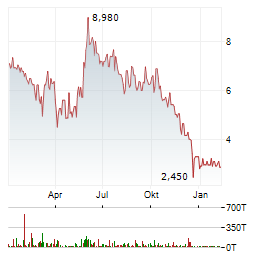
| DeFi Technologies Inc.: DeFi Technologies Announces Valour Receives UK Regulatory Approval and Begins Offering Select Yield-Bearing Crypto ETPs to UK Retail Investors via the London Stock Exchange | UK retail access now live: Valour has received approval from the FCA and the LSE and has begun offering select Valour ETPs to UK retail investors on the London... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu MOBIX LABS INC finden Sie auf Wallstreet Online.