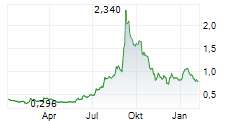
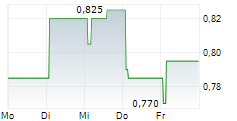
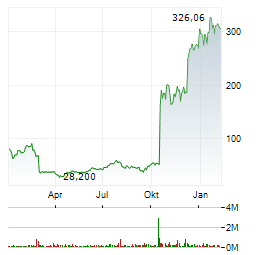
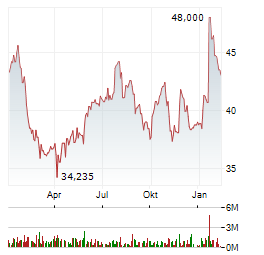
Chart-Typ:
Zeitraum:
| Praxis Precision Medicines, Inc.: Praxis Precision Medicines Appoints Global Epilepsy Leader Dr. Orrin Devinsky as Head of Clinical Strategy | BOSTON, Jan. 13, 2026 (GLOBE NEWSWIRE) -- Praxis Precision Medicines, Inc. (NASDAQ: PRAX), a fully integrated, leading central nervous system (CNS) precision neuroscience biopharmaceutical company... ► Artikel lesen |
| Praxis Precision Medicines, Inc.: Praxis Precision Medicines Further Positioned for Next-Phase Growth with Key Board and Executive Appointments | BOSTON, Jan. 08, 2026 (GLOBE NEWSWIRE) -- Praxis Precision Medicines, Inc. (NASDAQ: PRAX), a clinical-stage biopharmaceutical company translating genetic insights into the development of therapies... ► Artikel lesen |
| Praxis Precision Medicines, Inc.: Praxis Precision Medicines Announces the FDA Has Granted Breakthrough Therapy Designation for Ulixacaltamide HCl in Essential Tremor | The Breakthrough Therapy Designation was granted based on the positive topline results from the Essential3 Phase 3 program in essential tremor Praxis remains on track to submit ulixacaltamide NDA... ► Artikel lesen |
| Ionos, Kontron, Lanxess, Qiagen, Redcare Pharmacy, Siltronic, TeamViewer: Neue Aktien-Positionen der Short-Seller | Wer Aktien leerverkauft, also auf fallende Kurse spekuliert (Shortselling), unterliegt bestimmten Transparenzpflichten. Aktuelle Meldungen über Leerverkäufe geben Aufschluss darüber. Diese Vorgaben... ► Artikel lesen |
| DEUTSCHE BANK RESEARCH stuft QIAGEN NV auf 'Hold' | FRANKFURT (dpa-AFX Analyser) - Deutsche Bank Research hat die Einstufung für Qiagen mit einem Kursziel von 54 US-Dollar auf "Hold" belassen. Das vierte Quartal sei besser als erwartet gewesen, der Ausblick... ► Artikel lesen |
| ROUNDUP 2: Qiagen sieht sich für weiteres organisches Wachstum gut aufgestellt | (neu: Kurs) VENLO/HILDEN (dpa-AFX) - Der im Fokus von Übernahmespekulationen stehende Dax-Konzern Qiagen hat 2025 von einer anziehenden Nachfrage nach seinen Kernprodukten profitiert. Im neuen Jahr... ► Artikel lesen |
| Avidity Biosciences, Inc.: Avidity Biosciences Reports Third Quarter 2025 Financial Results and Recent Highlights | Announced Novartis agreement to acquire Avidity for total equity value of approximately $12 billion; Avidity expects to separate its early-stage precision cardiology... ► Artikel lesen |
| ROUNDUP/Aktien New York Schluss: Rekordrally läuft - Hoffen auf Zollstreit-Ende | NEW YORK (dpa-AFX) - In Erwartung einer baldigen Einigung im Zollstreit zwischen den USA und China ist die Rekordjagd an den US-Börsen am Montag weitergegangen. Technologiewerte waren die größten Profiteure... ► Artikel lesen |
| Aktien New York: Rekordrally läuft nach Entspannungssignal im Zollstreit weiter | NEW YORK (dpa-AFX) - Eine vorläufige Einigung im Zollstreit zwischen den USA und China hat den Rekordlauf der US-Börsen am Montag weiter angeheizt. Technologiewerte waren die größten Profiteure. Das... ► Artikel lesen |
Sie erhalten auf FinanzNachrichten.de kostenlose Realtime-Aktienkurse von und sowie Kurse und Daten von ARIVA.DE AG.
Weitere Kennzahlen, Fundamentaldaten und Unternehmensinformationen zu SIRNAOMICS LTD finden Sie auf Wallstreet Online.