
New digital image analysis data of stained tissues and biopsies with FibroNest is presented for the continuous assessment of the severity and regression of Fibrosis in human in-vitro 3D NASH Spheroid models and for the automated classification of post-transplant patients in F4 Fibrosis Stage with and without Hepatocellular carcinoma (HCC) at the Liver Meeting Digital Experience 2020
PRINCETON, NJ / ACCESSWIRE / November 11, 2020 / PharmaNest is an image analysis company focused on the development and validation of novel standards for the quantification of Fibrosis and associated histological features for drug discovery and development. Recently, PharmaNest announced the launch of its FibroNest cloud-based and multi-vendor platform for the automated quantification of Fibrosis for non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) from digital images of stained tissues and for research-only-use projects at this time.
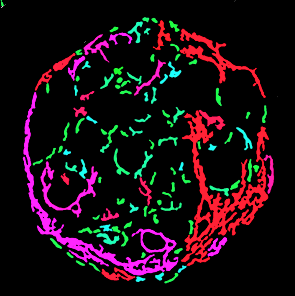
In human in-vitro 3D NASH tissue model, FibroNest
quantifies 32 phenotypic traits in 7 statistical
dimensions to describe and quantify the
progression of fibrosis, and regression with
drug treatment.
As a multi-vendor platform, FibroNest is compatible with all kinds of formats of digital images of tissues and biopsies digital images from conventional digital pathology stained slides (Picro Sirius Red, Trichrome, or Antibody stains for fibrosis) acquired by FDA-approved digital pathology slides scanners or other digital microscopes.
The Platform has been engineered to quantify the fibrosis phenotype in three phenotypic dimensions, including more than 40 traits and +350 parameters. The model has been validated to establish a continuous score for the assessment of the severity and progression of fibrosis in 20+ pre-clinical models and, most recently, in clinical PBC, Adult NASH, and Pediatric NASH. It remains an investigational tool at this stage. The Fibrosis-associated histological features such as Steatosis, lobular inflammation, and hepatocellular ballooning are also automatically quantified by FibroNest.
At the 2020 Liver Meeting, PharmaNest presents results that extend the translational capabilities of FibroNest to the NASH Spheroid Model in collaboration with InSphero (Schlieren, Switzerland), where FibroNest is used to quantify and describe the progression of fibrosis in human in-vitro 3D NASH tissue models and their response to treatment in a robust and reproducible way, showing the utility of the combined technologies to accelerate the discovery of novel anti-fibrotic compounds.
In collaborations with the Nagasaki University (Japan), PharmaNest shows that phenotypic analysis of collagen with FibroNest® from digital histopathology images is an automated method to classify HCC from non-HCC in patients who underwent Liver Transplant with severe fibrosis with superior sensitivity and specificity performance. This can be of clinical importance for the appropriate guidance of treatment strategy.
The results will be presented at The Liver Meeting Digital Experience (TLMdX) 2020, including the following poster presentations and schedule. Please contact PharmaNest to schedule other one-on-one or group presentations over webinar.
#0566: Novel phenotypic image analysis of 3D NASH model generates quantitative and continuous scores for the evaluation of fibrosis in vitro. Mathieu M. Petitjean1, Radina Kostadinova 2, Li Chen 1, Simon Str?bel 2, Eva Thoma2 (1) PharmaNest, Princeton, NJ, USA (2) InSphero AG, Schlieren, Switzerland
# 1481: Automated Fibrosis Phenotyping of NASH non-tumorous lesions Digital Images Helps Classify HCC and non-HCC NASH patients who underwent liver transplantation. Hisamitsu Miyaaki 1, Yuko Akazawa 1, Li Chen 2, Mathieu Petitjean 2 (1) Nagasaki University, Nagasaki, Japan (2) PharmaNest, Princeton, NJ, USA
PRESS CONTACT:
+1 (609) 375 2003
info@mednest.com
MEDNEST, LLC
Additional Links
www.fibronest.com
SOURCE: PharmaNest
View source version on accesswire.com:
https://www.accesswire.com/616242/PharmaNest-Announces-New-Pre-Clinical-NASH-and-Clinical-Liver-Cancer-Data-to-Be-Presented-at-the-AASLD-the-Liver-Meeting-Digital-ExperienceTM-TLMdX-2020
