LONDON, Oct. 7, 2021 /PRNewswire/ -- The Al Hikma Modern Hospital, Zarqa, Jordan, today announced it is the first hospital in the region to adopt the geko device to treat and prevent acute lower limb post-operative and trauma-based oedema for enhanced recovery.

Oedema, the medical term for swelling - a huge and, until now, silent burden with few tools to address the complication - can delay pre-operative surgical fixation, impede post-operative wound closure, decrease muscle strength and stall rehabilitation[1].
The current standard of care for pre-operative oedema reduction in ankle fracture patients is a backslab plaster cast in combination with leg elevation; and for post-operative elective surgeries, most often a boot-like cuff that compresses the lower limb to increase blood flow, called intermittent pneumatic compression (IPC).
IPC, however, is not suitable for all patients due to vascular disease, fragile skin, or complex limb injury[2]. IPC also requires resource to correctly fit the cuffs and the pneumatic pumps to inflate them are not always readily available - and elevation alone, can cause increased length of hospital stay and with it increased costs. The result is the need for an alternative mechanical intervention that can surpass IPC and leg elevation - a therapy able to reduce length of hospital stay, follow a patient home, facilitate enhanced recovery and allow rehabilitation to begin sooner.
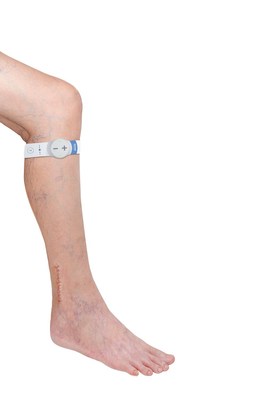
To address this significant unmet need, the orthopaedic department at the Al Hikma Hospital, led by Dr Sameih Ismail Abu Khaleifa, Consultant Orthopaedic Surgeon, has adopted Sky Medical Technology's geko device. A small watch-sized device that sticks to the side of the knee, increasing blood flow in the deep veins of the calf[3], via painless electrical pulses, at a rate equal to 60 percent of walking[4] without a patient having to move. Through this mechanism of non-invasive neuromuscular electrostimulation, the device prevents oedema build-up and enables oedema clearance by local reabsorption of oedematous fluid into the vasculature and lymphatic systems[5],[6].
Influenced by compelling NHS real-world data, reporting a pre-operative 2 day improvement in readiness for surgery in ankle fracture patients[5]; and an RCT (randomized control trial) demonstrating post-operative oedema prevention in hip replacement patients[6], Dr Sameih Ismail Abu Khaleifa saw the potential for the innovative geko device and embraced the opportunity to examine its role and subsequent adoption into clinical practice.
Dr Sameih Ismail Abu Khaleifa, Consultant Orthopedic Surgeon, said: "Lower limb post-operative and trauma-based oedema is a complicating factor that can impact length of hospital stay, functional recovery and readmission rates1. My team were therefore keen to explore geko device use as an alternative mechanical intervention to enhance recovery. The easy-to-use device, the size of a wrist-watch, is now in routine use providing safe and well-tolerated oedema control, ensuring that all of our patients can now receive appropriate oedema management for better clinical outcomes - and importantly improved patient satisfaction."
Bernard Ross, founder and CEO of Sky Medical Technology, said: "I am delighted that Dr Sameih Ismail Abu Khaleifa and his clinical team are the first in their region to champion use of the geko device to manage post-operative and trauma-based oedema, essential to optimizing the effectiveness of other interventions, such as physical therapy, that combined can enhance functional recovery. My thanks also goes to Tadawi Medical Supplies, importer and distributor of the geko device, for supporting innovation adoption in orthopaedics at the Al Hikma hospital."
References
- Loyd BJ, et al. Development of a reference chart to monitor postoperative swelling following total knee arthroplasty. Disabil Rehabil. 2020 Jun;42(12):1767-1774. doi: 10.1080/09638288.2018.1534005. Epub 2019 Jan 22.
- Eberhard R, et al. Risks and contraindications of medical compression treatment - A critical reappraisal. An international consensus statement. Phlebology. 2020 Aug;35(7):447-460. doi: 10.1177/0268355520909066. Epub 2020 Mar 2.
- A.Nicolaides, M Griffin. Measurement of blood flow in the deep veins of the lower limb using the geko neuromuscular electro-stimulation device. Journal of International Angiology August 2016-04.
- Tucker A, et al. Augmentation of venous, arterial and microvascular blood supply in the leg by isometric neuromuscular stimulation via the peroneal nerve. The International journal of angiology: official publication of the International College of Angiology, Inc. 2010 Spring; 19(1): e31-7.
- Mahmood et al, Neuromuscular Electrostimulation Device Reduces Preoperative Edema and Accelerates Readiness for Theater in Patients Requiring Open Reduction Internal Fixation for Acute Ankle Fracture the foot and ankle journal, published online, March 2020
- Wainwright TW et al. A Feasibility Randomised Controlled Trial to Evaluate the Effectiveness of a Novel Neuromuscular Electro-stimulation Device in Preventing the Formation of Oedema Following Total Hip Replacement Surgery. Heliyon 18 Jul 2018- Volume 4, Issue 7
About the geko device
The geko is a neuromuscular electrostimulation device that gently stimulates the common peroneal nerve with small electrical pulses, activating the calf and foot muscle pumps to increase blood flow in the deeps veins of the calf at a rate equal to 60% of walking without the patient having to move. In the circumstance of lower limb trauma, the geko reduces the net filtration of fluid leakage from the capillary bed. Through this unique mechanism of action, the geko device prevents oedema build-up and enables oedema clearance by local reabsorption of excess interstitial fluid into the vasculature and lymphatic systems. Weighing just 10g, silent in operation and with no wires or leads, the battery powered geko is a daily disposable device that is self-adhesive and comfortable to wear. www.gekodevices.com
About Firstkind and Sky Medical Technology Ltd
Sky Medical Technology, the parent of Firstkind Ltd, is a UK-based medical devices company. Through its innovative mechanism of neuromuscular electrostimulation, Sky has developed a non-invasive, ground-breaking technology platform, OnPulse, embedded in its industry-leading brand, the geko device. Sky's products are tailored to different medical application areas, selling through strategic partnerships or distributors in each major clinical area. Clinical areas of focus include life threatening blood clots, complications related to swelling after orthopaedic surgery and vascular conditions related to wound healing. The goal in each pathway is to partner with healthcare professionals to improve clinical outcomes and patient care whilst saving health system resources. www.skymedtech.com
About Tadawi Medical Supplies Est
Tadawi is a leading importer and distributor of medical equipment for the Jordanian market. Focus application areas include intensive care and wound therapy, alongside exclusive distribution agreements with leading international companies in the respiratory field. Their selling organization has a strong medical background, with staff drawn from medical device engineering, nursing and health education.
Photo - https://mma.prnewswire.com/media/1653496/T3_Device_3D_render_Flat.jpg
Photo - https://mma.prnewswire.com/media/1654698/The_geko__device_on_the_leg.jpg
Logo - https://mma.prnewswire.com/media/1340942/Sky_Medical_Logo.jpg
Media Contact
Sue Davenport
Hawk House
Peregrine Business Park
High Wycombe
Buckinghamshire
HP13 7DL
+44 (0)333 444 0468