
New Class of Navigated TMS Delivers Customized, Repeatable Treatments Improving Patient Care and Clinical Efficiencies
Magstim, the global leader in neuroscience research and treatment for mental health, has been awarded 510K clearance for Horizon 3.0. Designed specifically for the clinical setting, Horizon 3.0 offers enhanced workflows with navigated TMS and analytics.Magstim Connect is a new powerful patient data management system for TMS delivering connected care at networked sites.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20211102005137/en/
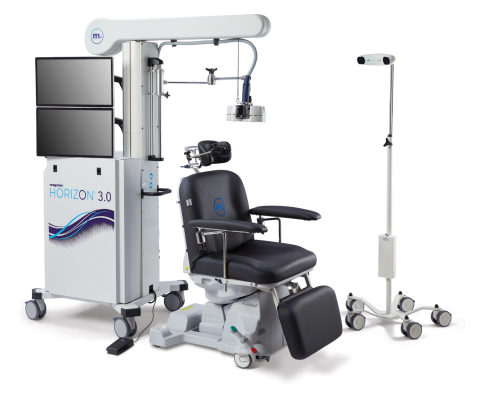
Magstim H3 is a new class of navigated transcranial magnetic stimulation (TMS) delivering customized, repeatable treatments, improving patient care and clinical efficiencies. Designed specifically for the clinical setting, Horizon 3.0 offers enhanced workflows with navigated TMS and analytics. Magstim Connect is a new powerful patient data management system for TMS delivering connected care at networked sites. TMS is a form of non-invasive brain stimulation that uses an electromagnetic coil to stimulate nerve cells in the region of the brain involved in mood control and depression. Magstim developed the first TMS system more than 30 years ago and there have been close to 16,000 published studies citing the use of Magstim Stimulators in neuroscientific and clinical research. This FDA clearance allows Magstim to bring Horizon 3.0 to thousands of physicians and clinicians in the USA. To learn more about Horizon 3.0 and Magstim research and clinical neurotechnology visit www.magstim.com (Photo: Business Wire).
"We spoke with physicians, patients, researchers and employees and asked them to imagine the next-generation of TMS technology," said Lothar Krinke, PhD, CEO Magstim. "How can we improve patient care efficacy and efficiencies? Horizon 3.0 is the culmination of Magstim's foundation in science, technology and our passion to improve patient outcomes."
Magstim developed the first TMS system more than 30 years ago and there have been close to 16,000 published studies citing the use of Magstim Stimulators in neuroscientific and clinical research.
TMS is a form of non-invasive brain stimulation that uses an electromagnetic coil to stimulate nerve cells in the region of the brain involved in mood control and depression.
Horizon 3.0 integrates Magstim StimGuide+, which adds new layers of quality control analytics and workflow improvements to the first FDA-cleared TMS navigation system designed for the clinical setting. Magstim Connect is an advanced new patient data management system for TMS.
"Our TMS patients have experienced a high-degree of success, allowing them to change their lives," said Khaled Bowarshi, M.D., Florida TMS Clinic. "We strive to provide the best technology for our patients. I can now with confidence crown Magstim's Horizon 3.0 with StimGuide+ to be the best TMS system on the market today."
This FDA clearance allows Magstim to bring Horizon 3.0 to thousands of physicians and clinicians in the USA.
To learn more about Horizon 3.0 and Magstim research and clinical neurotechnology innovations, visit Magstim.com or call 844-624-7846.
About Welcony
Globally, Welcony technologies have supported thousands of research labs, clinics, hospitals and universities that focus on mental health, brain disorders, cognitive neuroscience and neuromonitoring. Key brands include Magstim Magnetic Stimulation, MagstimEGI high-density EEG, Technomed Clinical Neurophysiology and Neurosign Interoperative Nerve Monitoring. Welcony is backed by Telegraph Hill Partners, a San Francisco based private equity company.
View source version on businesswire.com: https://www.businesswire.com/news/home/20211102005137/en/
Contacts:
Media Contact: Mark Sejvar, mark.sejvar@magstimegi.com, 323-363-3530
