- Neurology journal BRAIN publishes efficacy and safety results at 1.5-year post treatment
- BMJ1 Open Ophthalmology publishes characteristics of the ND4-LHON study population before treatment
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221116005984/en/
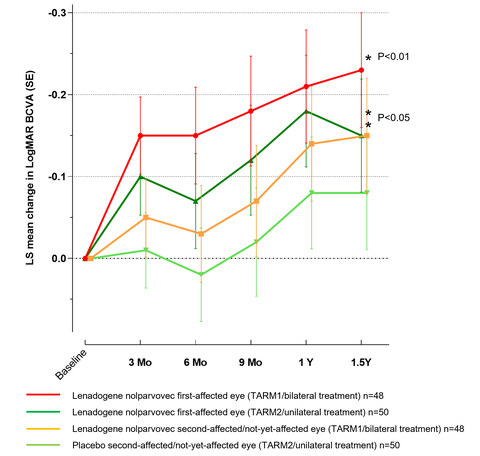
Figure 1: Time Course of LS Mean Change in LogMAR BCVA from Baseline to 1.5 Years for First-Affected and Second-Affected/Not-Yet-Affected Eyes Estimated by Linear Mixed Model (ITT Population) n=98. Note: The figure above shows results from a linear mixed model (considering both eyes of each patient) using treatment and baseline value as fixed effects, and intercept per patient as random effect. Data shown are LS means. (Graphic: Business Wire)
GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, today announced that the highly regarded neurology journal BRAIN has published efficacy and safety findings at 1.5 Year post-treatment in ND4-LHON patients treated with lenadogene nolparvovec (LUMEVOQ) from the REFLECT Pivotal clinical trial.
The REFLECT results, whose topline findings were announced by the company on 30th June 2021, show statistically significant visual acuity improvement in ND4-LHON patients from baseline in LUMEVOQ treated eyes, with an additional effect for bilaterally injected patients in comparison with a unilateral treatment (Figure 1). A good safety profile was observed and was comparable in unilaterally and bilaterally treated patients demonstrating the positive outcome of bilateral injections of LUMEVOQ
"These results demonstrate that LUMEVOQ produces a significant improvement in visual acuity for LHON patients, with a likely additional effect when patients are treated bilaterally," said Patrick Yu-Wai-Man2MD, PhD, Professor of Ophthalmology and Honorary Consultant Neuro-ophthalmologist at the University of Cambridge, Moorfields Eye Hospital, and the UCL Institute of Ophthalmology, United Kingdom, and Principal Investigator of REFLECT. "They also show that this efficacy is achieved with a good safety profile. This gene therapy represents a real hope for patients affected by this devastating blinding disease."
REFLECT is the third Phase III clinical trial evaluating the efficacy and safety of lenadogene nolparvovec in ND4-LHON patients. It is also the first clinical trial assessing the efficacy of a bilateral intravitreal injection (IVT) of LUMEVOQ, while in the three previous trials REVEAL1, REVERSE2,3,4 and RESCUE,3,4,5 it was exclusively administered as unilateral IVT. With the completion of REFLECT, LUMEVOQhas been administered to 189 patients across four clinical trials.
The paper, entitled "Randomised trial of bilateral injection of lenadogene nolparvovec for m.11778G>AMT-ND4 Leber hereditary optic neuropathy",is available in print and online via this link.
In addition, the BMJ Open Ophthalmology, an international peer-reviewed journal, published analysis of characteristics of the ND4-LHON patients before treatment from the REFLECT trial. The article, titled "Study Design and Baseline Characteristics for the REFLECT Gene Therapy Trial of m.11778G>A/ND4-LHON" is available online on this link.
The analyses of the REFLECT trial population before treatment show demographic characteristics, visual function, and retinal anatomic measurements consistent with the natural history of the ND4-LHON disease6
"The profile of REFLECT patients, viewed together with those of REVERSE and RESCUE, provide important information on the inferred natural history of ND4-LHON that should help guide future research in subjects with the m.11778G>A mutation," said Prem Subramanian, MD, PhD, Professor of Ophthalmology, Neurology, and Neurosurgery at the Sue Anschutz-Rodgers University of Colorado Eye Center and REFLECT Principal Investigator.
References
1 Vignal-Clermont C, Girmens JF, Audo I, et al. Safety of intravitreal gene therapy for treatment of subjects with Leber hereditary optic neuropathy due to mutations in the mitochondrial ND4 gene: the REVEAL study. Biodrugs. 2021;35(2):201-214.
2 Yu-Wai-Man P, Newman NJ, Carelli V, et al. Bilateral visual improvement with unilateral gene therapy injection for Leber hereditary optic neuropathy. Sci Transl Med. 2020;12(573):eaaz7423.
3 Moster ML, Sergott RC, Newman NJ, et al. Cross-sectional analysis of baseline visual parameters in subjects recruited into the RESCUE and REVERSE ND4-LHON gene therapy studies. J Neuroophthalmol. 2021;41(3):298-308.
4 Newman NJ, Yu-Wai-Man P, Carelli V, et al. Intravitreal gene therapy vs. natural history in patients with Leber hereditary optic neuropathy carrying the m.11778G>A ND4 mutation: systematic review and indirect comparison. Front Neurol. 2021;12:662838.
5 Newman NJ, Yu-Wai-Man P, Carelli V, et al. Efficacy and safety of intravitreal gene therapy for Leber hereditary optic neuropathy treated within 6 Months of disease onset. Ophthalmology. 2021;128(5):649-660.
6 Newman NJ, Carelli V, Taiel M, Yu-Wai-Man P. Visual outcomes in Leber hereditary optic neuropathy patients with the m.11778G>A (MTND4) mitochondrial DNA mutation. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc. 2020;40(4):547-557.
About GenSight Biologics
GenSight Biologics S.A. is a clinical-stage biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders. GenSight Biologics' pipeline leverages two core technology platforms, the Mitochondrial Targeting Sequence (MTS) and optogenetics, to help preserve or restore vision in patients suffering from blinding retinal diseases. GenSight Biologics' lead product candidate, LUMEVOQ (GS010; lenadogene nolparvovec), is an investigational compound and has not been registered in any country at this stage; a marketing authorization application is currently under review by the EMA for the treatment of Leber Hereditary Optic Neuropathy (LHON), a rare mitochondrial disease affecting primarily teens and young adults that leads to irreversible blindness. Using its gene therapy-based approach, GenSight Biologics' product candidates are designed to be administered in a single treatment to each eye by intravitreal injection to offer patients a sustainable functional visual recovery.
About LUMEVOQ (GS010; lenadogene nolparvovec)
LUMEVOQ (GS010; lenadogene nolparvovec) targets Leber Hereditary Optic Neuropathy (LHON) by leveraging a mitochondrial targeting sequence (MTS) proprietary technology platform, arising from research conducted at the Institut de la Vision in Paris, which, when associated with the gene of interest, allows the platform to specifically address defects inside the mitochondria using an AAV vector (Adeno-Associated Virus). The gene of interest is transferred into the cell to be expressed and produces the functional protein, which will then be shuttled to the mitochondria through specific nucleotidic sequences in order to restore the missing or deficient mitochondrial function. "LUMEVOQ" was accepted as the invented name for GS010 (lenadogene nolparvovec) by the European Medicines Agency (EMA) in October 2018.LUMEVOQ (GS010; lenadogene nolparvovec), is an investigational compound and has not been registered in any country at this stage; a marketing authorization application is currently under review by the EMA.
About REFLECT
REFLECT is a multi-center, randomized, double-masked, placebo-controlled study to evaluate the safety and efficacy of bilateral injections of GS010 in subjects with LHON due to the NADH dehydrogenase 4 (ND4) mutation. In the active arm, GS010 was administered as a single intravitreal injection in each eye of each subject. In the placebo arm, GS010 was administered as a single intravitreal injection to the first affected eye, while the fellow eye received a placebo injection.
The primary endpoint for the REFLECT trial is the BCVA reported in LogMAR at 1.5 years (78 weeks) post-treatment in the second-affected/not-yet-affected eye. The change from baseline in second-affected/not-yet-affected eyes receiving GS010 and placebo is the primary response of interest. The secondary efficacy endpoints include: BCVA reported in LogMAR at 2 years post-treatment in the second-affected/not-yet-affected eye compared to both placebo and the first-affected eye receiving GS010, OCT and contrast sensitivity and quality of life scales.
The trial was conducted in multiple centers across Europe (1 each in France, Spain, Italy and the UK), the US (6 centers) and Taiwan (1 center). The trial planned to enroll 90 subjects with vision loss up to 1 year in duration; 98 subjects were successfully screened and treated. The first subject was treated in March 2018 and the last one in July 2019.
ClinicalTrials.gov Identifiers:
REFLECT: NCT03293524
_______________________
1 British Medical Journal
2 Patrick Yu-Wai-Man:
- Cambridge Centre for Brain Repair and MRC Mitochondrial Biology Unit, Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK.
- Cambridge Eye Unit, Addenbrooke's Hospital, Cambridge University Hospitals, Cambridge, UK.
- Moorfields Eye Hospital, London, UK
- UCL Institute of Ophthalmology, University College London, London, UK
View source version on businesswire.com: https://www.businesswire.com/news/home/20221116005984/en/
Contacts:
GenSight Biologics
Corporate Communications Director
Clothilde Caillet
ccaillet@gensight-biologics.com
RooneyPartners
Media Relations
Jeanene Timberlake
jtimberlake@rooneypartners.com
+1 646-770-8858
LifeSci Advisors
Investor Relations
Guillaume van Renterghem
gvanrenterghem@lifesciadvisors.com
+41 (0)76 735 01 31
Orpheon Finance
Retail Investors
James Palmer
j.palmer@orpheonfinance.com
+33 (0)7 60 92 77 74
