- Sustained efficacy three years after injection: statistically significant visual acuity improvement from nadir
- Continuous benefits in bilaterally treated patients with +17 to +20 ETDRS letters improvement vs nadir at 3 years
- 62% of bilaterally treated patients off-chart at baseline move back to on-chart territory at 3 years
- Good safety profile confirmed at 3 years
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230312005028/en/
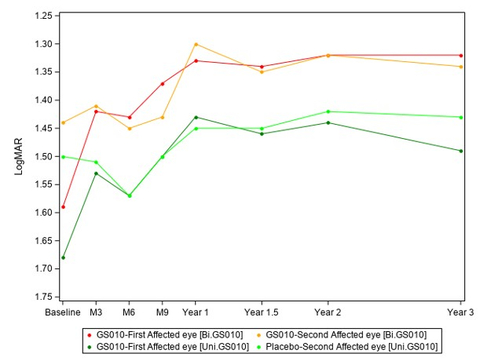
Graph 1: Evolution of Best-Corrected Visual Acuity (BCVA) over time (Graphic: Business Wire)
GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, today reported topline efficacy and safety results at 3 yearsa post-treatment administration in the REFLECT Phase III clinical trial with LUMEVOQ(lenadogene nolparvovec). The results show sustained efficacy and favorable safety for bilateral intravitreal injection of the gene therapy with a statistically significant visual acuity improvement from baseline in both treated eyes, showing an additional benefit of a bilateral injection compared to a unilateral injection.
"The REFLECT trial has delivered a further set of results demonstrating the sustained benefit of lenadogene nolparvovec, while reconfirming its safety profile saidNancy J Newman, MD, Professor of Ophthalmology and Neurology at Emory University School of Medicine (Atlanta, USA) and International Principal Investigator of REFLECT. In addition, the additional benefit among bilaterally treated patients suggests that injection of both eyes may be the best option for LHON patients with the ND4 mutation."
The findings reinforce the results observed at 2 years post-treatment administration, which were reported in December 20211, and at 1.5 years post-treatment administration, which were reported in June 2021.
Designed under a Special Protocol Assessment with the US FDA, REFLECT is a randomized, double-masked, placebo-controlled Phase III trial involving 98 subjects with vision loss due to Leber Hereditary Optic Neuropathy (LHON) caused by a mutated ND4 mitochondrial gene; enrolled ND4 subjects had vision loss up to one year from onset. Vision typically deteriorates to a nadir and stabilises to a level below the threshold for legal blindness. The ND4 mitochondrial mutation is associated with the most severe clinical form of LHON, with poor overall visual outcomes.1 All subjects received an intravitreal injection (IVT) of lenadogene nolparvovec in their first affected eye. The second affected eye was randomized to either a second IVT of LUMEVOQ or a placebo IVT, which was administered on the same day or the following day. 48 subjects were randomized to LUMEVOQ bilateral treatment, and 50 to lenadogene nolparvovec unilateral treatment (first-affected eye treated with lenadogene nolparvovec, second-affected eye treated with placebo).
______________________________
a Data cut-off: Nov 28, 2022
Sustained and meaningful efficacy at year 3
The evolution of the visual acuity over time shows that lenadogene nolparvovec effect is maintained over 3 years for all subjects, with an additional benefit with a bilateral injection as compared to a unilateral injection. Year 3 analyses confirm the dose effect that was noted at Year 1.5 and Year 2, with an absolute difference of the mean visual acuity between arms of +6,5 ETDRS letters in favor of bilaterally treated subjects at Year 3.
Graph 1: Evolution of Best-Corrected Visual Acuity (BCVA) over time
[Graph included above]
Baseline | M3 | M6 | M9 | Y1 | Y1.5 | Y2 | Y3 | |||||||||||||||||||
Number patients | 98 | 96 | 96 | 92 | 85 | 98 | 98 | 98 | ||||||||||||||||||
With LogMAR values non imputed | 0 | 2 | 2 | 6 | 13 | 0 | 0 | 0 | ||||||||||||||||||
With LogMAR values imputed | 0 | 0 | 0 | 0 | 0 | 25* | 20# | 28# |
Data cut-off: Nov. 28, 2022
Imputation LOCF of first available BCVA after theoretical V12 (after 518 days): clinical decisions taken at the blind data review committee before database lock and primary endpoint analysis; aligned with SAP
Imputation LOCF only: aligned with SAP
Note: imputed/non imputed data apply to both eyes per patient
Table 1: Change in Best-Corrected Visual Acuity (BCVA) versus Nadir 3 Years after Injection | ||||||
3-year | ||||||
1st affected eye | 2nd affected eye | |||||
Subjects
| LUMEVOQ -0.40 LogMAR
p<0.0001 | LUMEVOQ -0.33 LogMAR
p<0.0001 | ||||
Subjects
| LUMEVOQ -0.36 LogMAR
p<0.0001 | PLACEBO -0.28 LogMAR
p<0.0001 | ||||
Data cut-off: Nov 28, 2022. Subjects bilaterally treated: 1st affected eyes: n=48; 2nd affected eyes: n=48; subjects unilaterally treated: 1st affected eyes: n=50; 2nd affected eyes: n=50.
Comparison against nadir (i.e., the worst BCVA recorded from baseline to Year 3) starkly demonstrates the efficacy of lenadogene nolparvovec, even for the placebo eyes that improved via a contralateral treatment effect (also documented in sham-treated eyes in the REVERSE2 and RESCUE3 trials).
Responder analyses point to the benefits of treatment for patients that would otherwise have experienced significant vision loss with a very low likelihood of spontaneous recovery (partial recovery in 11% of patients described in the literature).1 Three years after a bilateral injection, 73% of patients had experienced a clinically meaningful improvement of at least -0.3 LogMAR (+15 ETDRS letters) relative to their observed nadir, and, among patients who were not able to read any letters on a screen at baseline (off-chart values for both eyes), 62% are now able to read letters.
Favorable safety profile
The favorable safety profile of lenadogene nolparvovec continued to be confirmed, with the safety profile of the drug being demonstrated as comparable in bilaterally and unilaterally treated subjects. There was no study discontinuation related to systemic or ocular adverse events, and there were no serious ocular adverse events. The main ocular adverse event was intraocular inflammation, which was mostly mild and responsive to conventional treatment.
REFLECT patients will be followed up to 5 years post-injection to monitor the efficacy and safety of lenadogene nolparvovec over time.
"We are very pleased to see that this 3 years' follow-up results from the REFLECT trial confirm the durable and meaningful efficacy of lenadogene nolparvovec, particularly for bilaterally treated patients," said Bernard Gilly, CEO and Co-Founder of GenSight Biologics. "The body of clinical evidence is making a compelling case that this gene therapy represents real hope for patients affected by this devastating blinding disease. These latest data will provide further significant support for the ongoing review of our dossier by the EMA."
References:
- Newman NJ, Carelli V, Taiel M, Yu-Wai-Man P. Visual outcomes in Leber hereditary optic neuropathy subjects with the m.11778G>A (MTND4) mitochondrial dna mutation. J Neuroophthalmol. (2020) 40:547-57. doi: 10.1097/WNO.0000000000001045.
- Yu-Wai-Man P, Newman NJ, Carelli V, Moster ML, Biousse V, Sadun AA, et al. Bilateral visual improvement with unilateral gene therapy injection for Leber hereditary optic neuropathy. Sci Transl Med. (2020) 12:eaaz7423. doi: 10.1126/scitranslmed.aaz7423
- Newman NJ, Yu-Wai-Man P, Carelli V, Moster ML, Biousse V, Vignal-Clermont C, et al. Efficacy and safety of intravitreal gene therapy for Leber hereditary optic neuropathy treated within 6 months of disease onset. Ophthalmology. (2021) 128:649-60. doi: 10.1016/j.ophtha.2020.12.012.
About GenSight Biologics
GenSight Biologics S.A. is a clinical-stage biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders. GenSight Biologics' pipeline leverages two core technology platforms, the Mitochondrial Targeting Sequence (MTS) and optogenetics, to help preserve or restore vision in patients suffering from blinding retinal diseases. GenSight Biologics' lead product candidate, LUMEVOQ® (GS010; lenadogene nolparvovec), is an investigational compound and has not been registered in any country at this stage; a marketing authorization application is currently under review by the EMA for the treatment of Leber Hereditary Optic Neuropathy (LHON), a rare mitochondrial disease affecting primarily teens and young adults that leads to irreversible blindness. Using its gene therapy-based approach, GenSight Biologics' product candidates are designed to be administered in a single treatment to each eye by intravitreal injection to offer patients a sustainable functional visual recovery.
About Leber Hereditary Optic Neuropathy (LHON)
Leber Hereditary Optic Neuropathy (LHON) is a rare maternally inherited mitochondrial genetic disease, characterized by the degeneration of retinal ganglion cells that results in brutal and irreversible vision loss that can lead to legal blindness, and mainly affects adolescents and young adults. LHON is associated with painless, sudden loss of central vision in the 1st eye, with the 2nd eye sequentially impaired. It is a symmetric disease with poor functional visual recovery. 97% of subjects have bilateral involvement at less than one year of onset of vision loss, and in 25% of cases, vision loss occurs in both eyes simultaneously. The estimated incidence of LHON is approximately 1,200-1,500 new subjects who lose their sight every year in the United States and the European Union.
About LUMEVOQ (GS010; lenadogene nolparvovec)
LUMEVOQ (GS010; lenadogene nolparvovec) targets Leber Hereditary Optic Neuropathy (LHON) by leveraging a mitochondrial targeting sequence (MTS) proprietary technology platform, arising from research conducted at the Institut de la Vision in Paris, which, when associated with the gene of interest, allows the platform to specifically address defects inside the mitochondria using an AAV vector (Adeno-Associated Virus). The gene of interest is transferred into the cell to be expressed and produces the functional protein, which will then be shuttled to the mitochondria through specific nucleotidic sequences in order to restore the missing or deficient mitochondrial function. "LUMEVOQ" was accepted as the invented name for GS010 (lenadogene nolparvovec) by the European Medicines Agency (EMA) in October 2018. LUMEVOQ® (GS010; lenadogene nolparvovec), is an investigational compound and has not been registered in any country at this stage; a marketing authorization application is currently under review by the EMA.
About REFLECT
REFLECT is a multi-center, randomized, double-masked, placebo-controlled study to evaluate the safety and efficacy of bilateral injections of GS010 in subjects with LHON due to the NADH dehydrogenase 4 (ND4) mutation. In the active arm, GS010 was administered as a single intravitreal injection in each eye of each subject. In the placebo arm, GS010 was administered as a single intravitreal injection to the first affected eye, while the fellow eye received a placebo injection.
The primary endpoint for the REFLECT trial is the BCVA reported in LogMAR at 1.5 years (78 weeks) post-treatment in the second-affected/not-yet-affected eye. The change from baseline in second-affected/not-yet-affected eyes receiving GS010 and placebo is the primary response of interest. The secondary efficacy endpoints include: BCVA reported in LogMAR at 2 years post-treatment in the second-affected/not-yet-affected eye compared to both placebo and the first-affected eye receiving GS010, OCT and contrast sensitivity and quality of life scales.
The trial was conducted in multiple centers across Europe (1 each in France, Spain, Italy and the UK), the US (6 centers) and Taiwan (1 center). The trial planned to enroll 90 subjects with vision loss up to 1 year in duration; 98 subjects were successfully screened and treated. The first subject was treated in March 2018 and the last one in July 2019.
ClinicalTrials.gov Identifiers:
REFLECT: NCT03293524
View source version on businesswire.com: https://www.businesswire.com/news/home/20230312005028/en/
Contacts:
GenSight Biologics
Chief Financial Officer
Thomas Gidoin
tgidoin@gensight-biologics.com
+33 (0)1 76 21 72 20
RooneyPartners
Media Relations
Jeanene Timberlake
jtimberlake@rooneypartners.com
+1-646-770-8858
LifeSci Advisors
Investor Relations
Guillaume van Renterghem
gvanrenterghem@lifesciadvisors.com
+41 (0)76 735 01 31
Orpheon Finance
Retail Investors
James Palmer
j.palmer@orpheonfinance.com
+33 (0)7 60 92 77 74
