First-Time Clinical Trial Outcomes Show Promising Follow-Up Results
HANGZHOU, China, June 19, 2023 /PRNewswire/ -- On May 17th, the 12-month results of the DRAGONFLY-DMR pivotal trial were presented at EuroPCR 2023. This trial was led by Professor Jian'an Wang from Zhe'er Hospital, Hangzhou, China, and presented on his behalf by the Eligibility Committee Chair, Professor Scott Lim, of the University of Virginia. The study met its pre-specified primary efficacy endpoint with a significant clinical success rate, contributing to increasing evidence supporting the safety and efficacy of Transcatheter-Edge-to-Edge Repair (TEER) therapy, and specifically the DragonFly valve repair system, for treating patients with chronic symptomatic 3+ to 4+ Degenerative Mitral Regurgitation (DMR) at prohibitive surgical risk.
Background
Severe Degenerative Mitral Regurgitation (DMR) is a prevalent heart valve disease that portends poor prognosis to patients if left untreated. Although surgical treatment is the gold standard for low surgical risk patients with DMR, a considerable number of patients worldwide remain undertreated due to high surgical risks or other factors. In recent years, TEER has emerged as the preferred technique for treating DMR in patients deemed high-risk for surgery, supported by a growing body of clinical evidence. Among the innovative TEER devices available, The DragonFly transcatheter mitral valve repair system stands out as a de-novo solution for DMR treatment.
Design
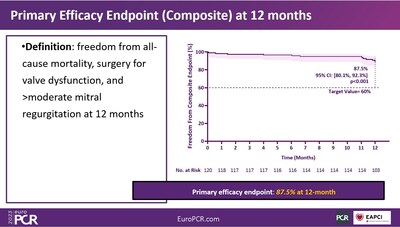
The study was conducted as a prospective, single-arm, multi-center clinical trial to evaluate the safety and effectiveness of the DragonFly device in treating symptomatic DMR (MR=3+) patients at prohibitive surgical risk. The sample size was 120 and the primary endpoint was the clinical success rate, which measured the freedom from all-cause mortality, mitral valve reintervention, and MR > 2+ at 1 year follow-up.
Results
Between May 2021 and January 2022, a total of 120 patients were enrolled in 27 medical centers across China. The mean age of the patients was 74.9±5.7 years, of which 49.2% were female. In all patients at enrollment, the baseline degree of MR was adjudicated as moderate-severe to severe by an independent echocardiographic core laboratory.
The immediate procedure success rate was both 99.2%. The composite success rate, defined as freedom from mortality, mitral valve surgery, or =2+ residual MR at 12 months with the DragonFly device was 87.5% (95% Confidence Interval: 80.1%, 92.3%). The device achieved the pre-determined primary efficacy endpoint.
The patients achieving MR=2+ was 90.4% at 1 month and 92.0% at 1 year. These findings indicate that the MR reduction by DragonFly is durable over that time frame.
The mean number of DragonFly devices implanted was 1.5±0.6. The mean time for the procedure and the device implantation time was 116.7±51.3 minutes and 96.6±47.7 minutes, respectively, taken into account that the TEER technique is new to the physicians in China. The mean mitral inflow gradient post-procedure and at 12 months were 2.8±1.3 mmHg and 3.2±1.4 mmHg, respectively.
Over time, left ventricular reverse remodeling was observed (p<0.05), along with significant improvements in the patients' functional and quality-of-life outcomes shown by increased New York Heart Association class I-II from 32.4% at baseline to 93.6% at 12 months (p<0.001) and Kansas City Cardiomyopathy Questionnaire of 31.1±18.2 from baseline (44.89±18.37) to 12 months (75.40±10.83) (p<0.001).
Conclusion
The results of this study demonstrate the high safety and efficacy of the DragonFly device for the treatment of DMR. The device's design and operational performance have led to sustained improvement in MR and left ventricular reverse remodeling, resulting in significant enhancements in the patients' cardiac function and quality of life.
DragonFly was independently designed and developed by Valgen Medtech and has been successfully implanted in over 300 patients in China (DMR/FMR/TR).
DragonFly will be the first domestic trans-femoral TEER product launched in China. It is anticipated that the device will soon become available in other parts of the world, providing new treatment option for patients with MR globally.
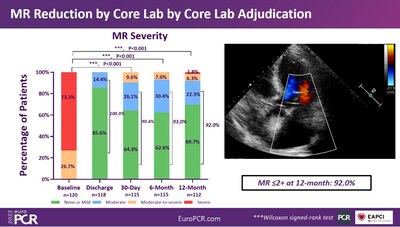
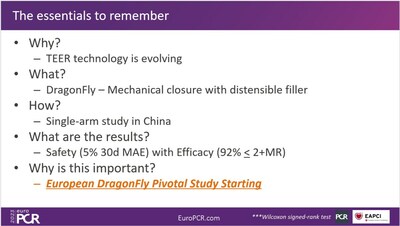

Photo - https://mma.prnewswire.com/media/2091682/image_1.jpg
Photo - https://mma.prnewswire.com/media/2091683/image_2.jpg
Photo - https://mma.prnewswire.com/media/2091684/image_3.jpg
Logo - https://mma.prnewswire.com/media/2091681/Valgen_Medtech_Logo.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/novel-transcatheter-valve-repair-device-dragonfly-dmr-12-month-trial-results-revealed-at-europcr-2023-301853995.html
View original content:https://www.prnewswire.co.uk/news-releases/novel-transcatheter-valve-repair-device-dragonfly-dmr-12-month-trial-results-revealed-at-europcr-2023-301853995.html

