
- Cash runway anticipated to be extended from Q2 2025 to Q4 2025
- Data from multiple Phase 1 and 2 clinical trials expected in 2024
OXFORD, United Kingdom, Jan. 05, 2024 (GLOBE NEWSWIRE) -- Barinthus Biotherapeutics plc (NASDAQ: BRNS), formerly Vaccitech plc, today provided a preliminary financial update and announced its 2024 corporate objectives. Barinthus Bio is a clinical-stage biopharmaceutical company developing novel T cell immunotherapeutic candidates designed to guide the immune system to overcome chronic infectious diseases, autoimmunity, and cancer.
"2024 promises to be another exciting year for Barinthus Bio, with multiple data readouts expected across our hepatitis B virus (HBV) infection, human papillomavirus (HPV) infection and prostate cancer programs, as well as the planned initiation of the first in human study of our SNAP platform-based candidate VTP-1000 in Celiac Disease," said Gemma Brown, Chief Financial Officer of Barinthus Bio. "Given the encouraging preliminary data in HBV and HPV infection and to maximise value for our stakeholders, we are focusing resources and deferring the planned IND application for VTP-1100 in HPV cancer. As a result of this, as well as the strategic phasing of manufacturing across our pipeline to focus on progressing our Phase 2 trials, we anticipate that our cash runway has extended from Q2 2025 to Q4 2025."
Financial Update:
- Cash and cash equivalents expected to be $142 million as of December 31, 2023.1
- Based on management's current assumptions, we believe our cash and cash equivalents will fund our operations into the fourth quarter of 2025.
- As of December 31, 2023, there were approximately 38.6 million ordinary shares issued and outstanding.
Anticipated 2024 Corporate Milestones:
H1 2024:
- Initiate a Phase 1 clinical trial with VTP-1000 in Celiac Disease.
- Announce final results from participants receiving VTP-200 in the Phase 1b/2 APOLLO (HPV001) trial evaluating the safety, immunogenicity and efficacy of VTP-200 in persistent HPV infection and low grade cervical lesions.
- Announce interim data from HBV003, our Phase 2b trial evaluating additional dosing of VTP-300 and PD-1 inhibition timing in people with chronic hepatitis B infection.
- Announce interim data from the first two arms of the Phase 2a AB-729-202 clinical trial evaluating VTP-300, NUC therapy and Arbutus' imdusiran in people with chronic hepatitis B infection.
H2 2024:
- Announce interim data from the third arm of the Phase 2a AB-729-202 clinical trial evaluating VTP-300, NUC therapy, Arbutus' imdusiran and nivolumab (Opdivo®) in people with chronic hepatitis B infection.
Barinthus Bio Product Pipeline:
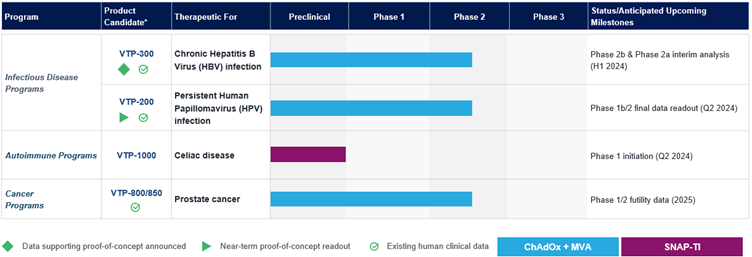
* Barinthus Bio has worldwide rights for all product candidates.
Partnered Pipeline:
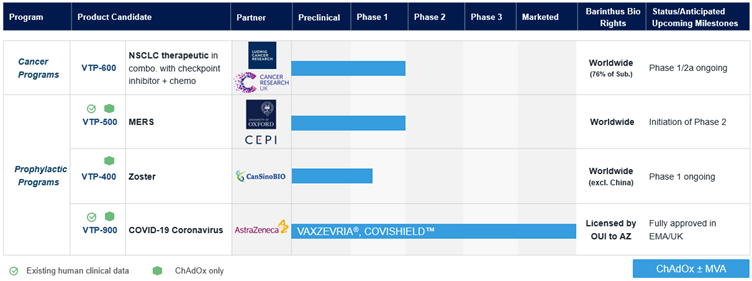
1 The preliminary estimate of cash and cash equivalents reflect management's current views and and may change as a result of management's review of results and other factors, including a wide variety of significant business, economic and competitive risks and uncertainties. Such preliminary financial information is subject to the finalization and closing of the accounting books and records of Barinthus Bio (which have yet to be performed) and should not be viewed as a substitute for full audited financial statements prepared in accordance with U.S. GAAP. In the course of preparing and finalizing the financial statements for the year ended December 31, 2023, the preliminary estimates of cash and cash equivalents for the year ended December 31, 2023 will be subject to change and Barinthus Bio may identify items that will require it to make adjustments to its preliminary estimates of its cash and cash equivalents. Any such changes could be material. For these or other reasons, the preliminary estimates of Barinthus Bio's cash and cash equivalents for the year ended December 31, 2023 may not ultimately be indicative of its results for such period and actual results may differ materially. No independent registered public accounting firm has audited, reviewed or compiled, examined or performed any procedures with respect to these preliminary estimated results, nor have they expressed any opinion or any other form of assurance on these preliminary estimated results.
About Barinthus Biotherapeutics
Barinthus Bio is a clinical-stage biopharmaceutical company developing novel T cell immunotherapeutic candidates designed to guide the immune system to overcome chronic infectious diseases, autoimmunity, and cancer. Helping people living with serious diseases and their families is the guiding principle at the heart of Barinthus Bio. With a broad pipeline, built around three proprietary platform technologies: ChAdOx, MVA and SNAP; Barinthus Bio is advancing a pipeline of four product candidates across a diverse range of therapeutic areas, including: VTP-300, an immunotherapeutic candidate designed as a potential component of a functional cure for chronic HBV infection; VTP-200, a non-surgical product candidate for persistent high-risk HPV infection; VTP-1000, an autoimmune candidate designed to utilize the SNAP-Tolerance Immunotherapy (TI) platform to treat patients with celiac disease; and VTP-850, a second-generation immunotherapeutic candidate designed to treat recurrent prostate cancer. Barinthus Bio's proven scientific expertise, diverse portfolio and focus on pipeline development uniquely positions the company to navigate towards delivering treatments for people with infectious diseases, autoimmunity and cancers that have a significant impact on their everyday lives. For more information, visit www.barinthusbio.com.
Forward Looking Statements
This press release contains forward-looking statements regarding Barinthus Bio within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, which can generally be identified as such by use of the words "may," "will," "plan," "forward," "encouraging," "believe," "potential," and similar expressions, although not all forward-looking statements contain these identifying words. These forward-looking statements include, without limitation, express or implied statements regarding our future expectations, plans and prospects, including our product development activities and clinical trials, including timing for readouts of any interim data for any of our programs, our anticipated regulatory filings and approvals, our preliminary estimated cash and cash equivalents, our cash runway, and our ability to develop and advance our current and future product candidates and programs. Any forward-looking statements in this press release are based on our management's current expectations and beliefs and are subject to numerous risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this press release, including, without limitation, risks and uncertainties related to the success, cost and timing of our pipeline development activities and planned and ongoing clinical trials, our ability to execute on our strategy, regulatory developments, the risk that we may not realize the benefits related to our rebranding and name change, our ability to fund our operations and access capital, our preliminary estimates of our cash and cash equivalents and cash runway, including the risk that final financial results may differ materially from our preliminary estimates, global economic uncertainty, including disruptions in the banking industry, the conflict in Ukraine, the conflict in Israel and Gaza, and other risks identified in our filings with the Securities and Exchange Commission (the "SEC"), including our Annual Report on Form 10-K for the year ended December 31, 2022, our Quarterly Reports on Form 10-Q and subsequent filings with the SEC. We caution you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. We expressly disclaim any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements.
IR contacts:
| Christopher M. Calabrese? Managing Director? LifeSci Advisors +1 917-680-5608 ccalabrese@lifesciadvisors.com? | Kevin Gardner Managing Director LifeSci Advisors +1 617-283-2856 kgardner@lifesciadvisors.com |
Media contact:
Audra Friis
Sam Brown, Inc.
+1 917-519-9577
audrafriis@sambrown.com ?
? ?
Company contact:
Jonothan Blackbourn
IR & PR Manager
Barinthus Bio
IR@barinthusbio.com
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/89f55abc-269f-40fa-8fbf-c05226ddcaf8
https://www.globenewswire.com/NewsRoom/AttachmentNg/f883add6-9c5e-4e17-a1c9-693ead20a6ff

