
SEOUL, South Korea, Jan. 29, 2024 /PRNewswire/ -- Daewoong Pharmaceutical's Bersiporocin (DWN12088) has been recognized as a significant advancement in treating idiopathic pulmonary fibrosis (IPF), a rare and debilitating disease. This first-in-class PRS inhibitor has recently received 'Orphan Drug Designation (ODD)' from the European Medicines Agency (EMA), adding to its earlier designation by the U.S. FDA for both IPF and systemic sclerosis.
The EMA's recognition highlights Bersiporocin's potential to meet critical needs in rare diseases, targeting conditions affecting fewer than 5 in 10,000 individuals in the EU. The designation offers several benefits, including guidance on clinical trials, reduced authorization fees, and 10-year market exclusivity upon approval.
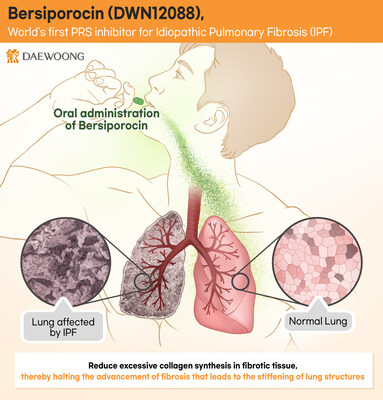
IPF is characterized by abnormal collagen accumulation in the lungs, reducing lung function. With a global incidence rate of about 13 cases per 100,000 people and a 5-year survival rate of only 40%, there's a pressing need for more effective and safer treatments.
Bersiporocin's mechanism involves inhibiting PRS (Prolyl-tRNA Synthetase), thus targeting the excess collagen production that leads to fibrosis. The drug's efficacy was demonstrated in a study published by the European Molecular Biology Organization (EMBO).
In addition to orphan drug designations, Bersiporocin has been granted fast track designation by the FDA. It also has been recognized in the 'Clinical Development Support' program by the Korea Drug Development Fund (KDDF). Bersiporocin has successfully completed phase 1 clinical trials in Korea and Australia, establishing its safety and pharmacokinetic properties in 162 healthy subjects. Multinational phase 2 clinical trials are currently in progress in Korea and the U.S.
Seng-ho Jeon, CEO of Daewoong Pharmaceutical, stated, "With Bersiporocin's global recognition and its clinical advancements, we are more committed than ever to delivering this promising treatment to IPF patients, potentially transforming the landscape of this challenging disease."
Forward-Looking Statements
This press release contains forward-looking statements that are based on the current beliefs and expectations of Daewoong Pharmaceutical's management. Factors that could cause or contribute to such differences include, but are not limited to: (1) Regulatory and governmental approvals: The approval process for pharmaceutical products is subject to extensive regulations and may involve uncertainties and delays. Any failure to obtain necessary approvals or the occurrence of delays in the approval process could adversely affect Daewoong Pharmaceutical's business and results of operations; and (2) Clinical trials: The success of Daewoong Pharmaceutical's products depends on the results of clinical trials. The results of early clinical trials may not be indicative of the results of later-stage or larger-scale clinical trials.

Photo - https://mma.prnewswire.com/media/2328539/Bersiporocin.jpg
Logo - https://mma.prnewswire.com/media/1676530/4517166/Daewoong_Pharmaceuticals_Logo.jpg
![]() View original content to download multimedia:https://www.prnewswire.co.uk/news-releases/daewoong-pharmaceuticals-bersiporocin-receives-orphan-drug-designation-in-europe-to-treat-idiopathic-pulmonary-fibrosis-302046478.html
View original content to download multimedia:https://www.prnewswire.co.uk/news-releases/daewoong-pharmaceuticals-bersiporocin-receives-orphan-drug-designation-in-europe-to-treat-idiopathic-pulmonary-fibrosis-302046478.html


