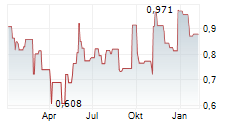
STOCKHOLM, Feb. 7, 2024 /PRNewswire/ -- Moberg Pharma ABs (OMX: MOB) marker partner in Sweden - Allderma AB, a company which specialises in the sale of over-the-counter (OTC) pharmaceuticals - has now launched sales of MOB-015 under the Terclara® brand. The interest among pharmacies has been significant for Moberg Pharma's medication against nail fungus.
" t is incredibly rewarding to work with such a committed market partner as Allderma", says Anna Ljung, CEO of Moberg Pharma, and continues:" The introduction of Terclara® to Swedish pharmacy chains has gone very well. Allderma's approach and good results demonstrate why they are an excellent partner in our home market." Allderma is managed by the commercial leaders which were responsible for the successful Nordic launch of Nalox®, Moberg Pharma's first-generation nail fungus product.
"We have high expectations for the launch of MOB-015 - the first topical medication with an equivalent antifungal effect to tablets but without the risk of serious side effects. The goal is the same as last time, to achieve a market-leading position", says Mimmi Frölén, CEO of Allderma AB.
Currently, the majority of pharmacies throughout Sweden have decided to start selling Terclara®.
For additional information, please contact:
Anna Ljung, CEO, telephone: +46 707 66 60 30, E-mail: anna.ljung@mobergpharma.se
Anders Bröijersén, Chief Medical Officer, telephone: +46 760 01 15 76, E-mail: anders.broijersen@mobergpharma.se
About this information
This information was submitted for publication, through the agency of the contact persons set out above, on February 7th, 2024, at 8.00 am CEST.
About MOB-015 and Onychomycosis
Approximately 10% of the general population suffer from onychomycosis and a majority of those afflicted go untreated. The global market opportunity is significant with more than hundred million patients worldwide and a clear demand for better products. Moberg Pharma estimates the annual worldwide peak sales potential for MOB-015 to be in the range of USD 250-500 million.
MOB-015 is an in-house developed topical formulation of terbinafine, enabling effective concentrations of terbinafine to the nail and nail bed while avoiding the risk of systemic exposure seen with oral terbinafine use. Oral terbinafine is currently the gold standard for treating onychomycosis but associated with safety issues, including drug interactions and liver damage. MOB-015 is recommended for national approval in 13 European countries and is launched in Sweden under the brand name Terclara®. The approval is supported by two Phase 3 trials where MOB-015 demonstrated superior levels of mycological cure (76% vs up to 42% for comparators), and a significantly better complete cure rate compared to vehicle, without any serious adverse reactions.
About Moberg Pharma, www.mobergpharma.com
Moberg Pharma AB (publ) is a Swedish pharmaceutical company focused on commercializing proprietary innovations based on drug delivery of proven compounds. The Company's asset, MOB-015, is a novel topical treatment for onychomycosis, for which market approvals in several EU-countries has recently been obtained. Data from phase 3 clinical trials in more than 800 patients for MOB-015 indicate that the product has the potential to become the future market leader in onychomycosis. Moberg Pharma has agreements with commercial partners in place in various regions including Europe and Canada. Moberg Pharma is headquartered in Stockholm and the Company's shares are listed on the Small Cap list of the Nasdaq Stockholm (OMX: MOB).
About Allderma Pharmaceuticals, www.allderma.se
Allderma Pharmaceuticals is a Swedish pharmaceutical company that since 2001 develops, sells and markets over the counter and self-care products. The range spans a number of different treatment areas, with a focus and expertise in dermatology. Founder Hans Svartholm started Allderma after working in the pharmaceutical industry for many years. The idea was simple and still applies it should be easy to find an effective and safe product for the most common skin problems. Allderma emphasis and invests in information for healthcare providers, pharmacists, doctors and consumers. Product specialists work continuously to be a spear head in research and innovation within dermatology. Allderma has been part of 3iM Invest AB's investment area Consumer Healthcare since 2020.
The following files are available for download:
https://mb.cision.com/Main/1662/3923400/2586205.pdf | MOB-015 is launched in Sweden under the brand name Terclara'" pharmacies report major demand for the new medication |
![]() View original content:https://www.prnewswire.co.uk/news-releases/mob-015-is-launched-in-sweden-under-the-brand-name-terclara-pharmacies-report-major-demand-for-the-new-medication-302055674.html
View original content:https://www.prnewswire.co.uk/news-releases/mob-015-is-launched-in-sweden-under-the-brand-name-terclara-pharmacies-report-major-demand-for-the-new-medication-302055674.html