
Soquelitinib advancing towards initial enrollment for registrational Phase 3 clinical trial for PTCL and randomized Phase 1 clinical trial for atopic dermatitis
Orphan drug designation and new interim data from Phase 1/1b clinical trial further demonstrate soquelitinib's potential to address the need for new treatments for PTCL
Conference call today at 4:30 p.m. ET / 1:30 p.m. PT
BURLINGAME, Calif., March 19, 2024 (GLOBE NEWSWIRE) -- Corvus Pharmaceuticals, Inc. (Corvus or the Company) (Nasdaq: CRVS) (GLOBAL NEWSWIRE), a clinical-stage biopharmaceutical company, today provided a business update and reported financial results for the fourth quarter and year ended December 31, 2023.
"Corvus is pioneering the development of ITK inhibition with a focus on advancing our lead program, soquelitinib, into a registrational Phase 3 trial for patients with relapsed PTCL," said Richard A. Miller, M.D., co-founder, president and chief executive officer of Corvus. "We believe we are well positioned to execute on this trial, with recent positive updates from our Phase 1/1b clinical trial, alignment on the final study protocol with FDA, interest from leading academic centers in North America, and the receipt of Orphan Drug Designation for soquelitinib. We also continue to expand our knowledge and experience with ITK-mediated immunomodulation as a novel therapeutic for a broad range of cancers and immune diseases. We have several exciting opportunities in immunology, including plans for a randomized, placebo controlled clinical trial of soquelitinib in atopic dermatitis. In addition, we continue to progress our next-generation ITK inhibitors, which have been designed to optimize T cell modulation for specific immune indications. Based on current timelines, we anticipate the atopic dermatitis trial will be initiated in the second quarter of 2024 with initial data potentially available by year end, and we anticipate the PTCL trial will be initiated in the third quarter."
Business Update and Strategy
Prioritized Program: Soquelitinib (formerly CPI-818, Corvus' selective ITK inhibitor)
Soquelitinib for T Cell Lymphoma
- Corvus continues to follow patients with relapsed T cell lymphoma in a Phase 1/1b clinical trial evaluating single agent therapy with soquelitinib. Updated interim data as of January 22, 2024 (data shown below in Figures 1-2):
- A total of 23 patients were enrolled in the Phase 1/1b trial at the optimum 200 mg two-times a day dose and meet the eligibility criteria for the planned registrational Phase 3 clinical trial based on =1 and =3 prior therapies (eligible patient population), including 21 evaluable patients.
- For the 21 evaluable patients, objective responses (complete response, CR plus partial response, PR) were seen in seven patients, including four CRs and three PRs. Compared to the prior data (as of November 21, 2023) reported from the trial in December 2023 in conjunction with the 65th American Society of Hematology (ASH) Annual Meeting and Exposition, one of the patients achieving a PR continued to respond and achieved a CR.
- Disease control (CR, PR and stable disease) was seen in 12 of 21 patients. The stable disease group included five patients who achieved tumor reductions that did not meet the criteria for a PR, with two of these patients continuing to receive therapy. Several patients experiencing tumor regression are continuing to receive therapy.
- The Company presented a poster at ASH that included additional data from the Phase 1/1b clinical trial and complementary preclinical data, including the evaluation of blood samples and tumor biopsies from eight patients who participated in the trial. The results support soquelitinib's novel mechanism of action, and demonstrated increases in cytotoxic killer T cells and reductions in markers of T cell exhaustion.
Figure 1: Waterfall Plot for Patients in the 200 mg Dose Cohort of the Soquelitinib Phase 1/1b Clinical Trial for Peripheral T Cell Lymphoma. The plot shows the best percent change in tumor volume in the 21 evaluable patients (eligible patient population), as of January 22, 2024, that were measurable by CT scan or by Modified Severity-Weighted Assessment Tool (mSWAT) for patients with cutaneous involvement.
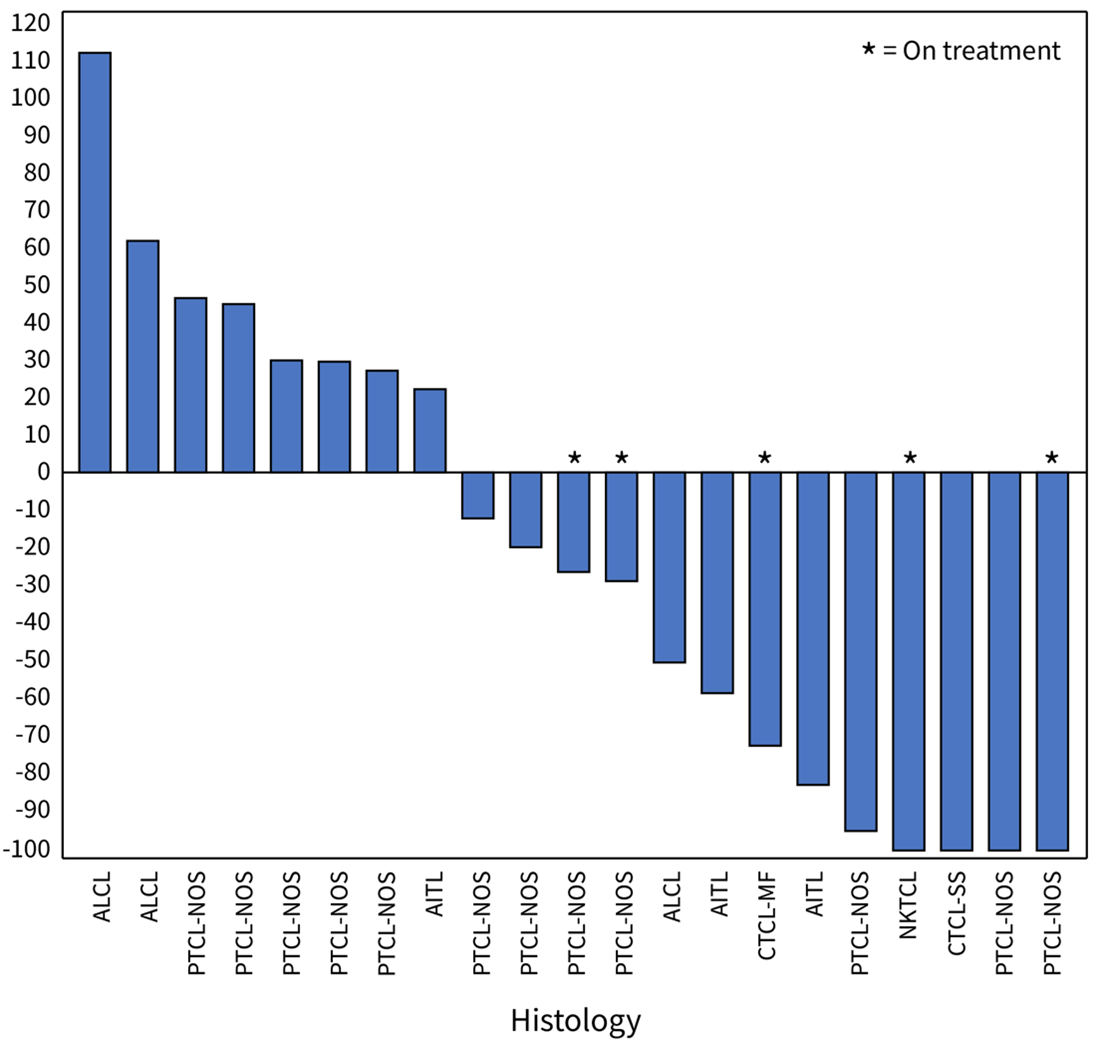
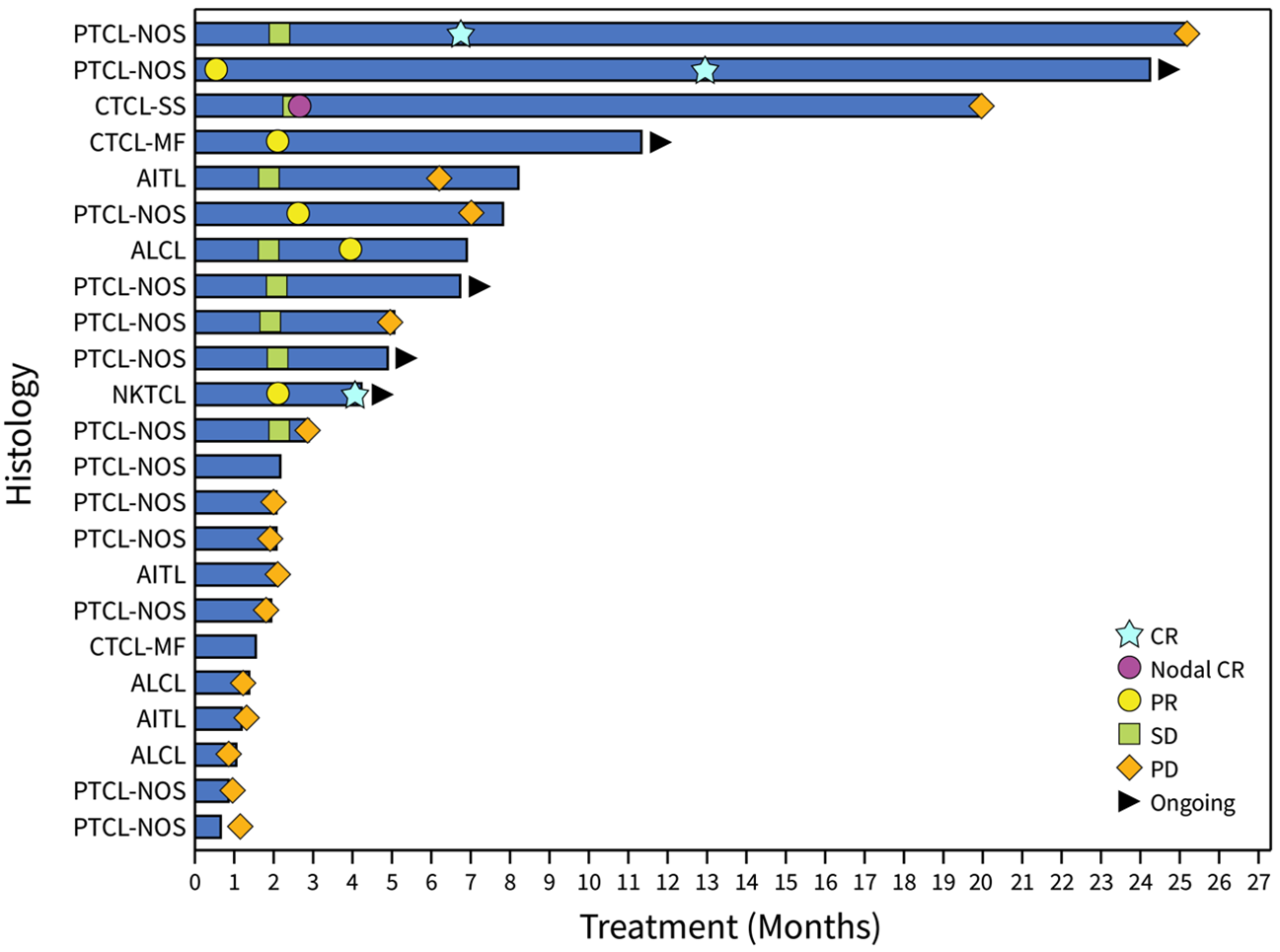
Figure 2: Swimmer Plot of Eligible Patient Population Demonstrating Response and Time on Therapy. Tumor histologies, as of January 22, 2024, are also shown indicating different types of T cell lymphoma. PTCL-NOS, peripheral T cell lymphoma not otherwise specified; CTCL, cutaneous T cell lymphoma of either Sezary or mycosis fungoides type; NKTCL, natural killer cell T cell lymphoma; ALCL, anaplastic large cell lymphoma; AITL, angioimmunoblastic T cell lymphoma.
- In February 2024, the U.S. Food and Drug Administration (FDA) granted Orphan Drug Designation for soquelitinib for the treatment of T cell lymphoma, providing potential benefits including assistance in the drug development process, tax credits for clinical costs, exemptions from certain FDA fees and seven years of post-approval marketing exclusivity. The Company has also obtained alignment with FDA on its protocol for a registrational Phase 3 clinical trial of soquelitinib in patients with relapsed PTCL and anticipate initiating the trial in the third quarter of 2024. There are currently no FDA fully approved agents for the treatment of relapsed PTCL.
Soquelitinib for Immune Diseases
- Corvus plans to initiate a randomized, placebo-controlled Phase 1 trial of soquelitinib in patients with moderate to severe atopic dermatitis in the second quarter of 2024, with the potential for initial data from the trial before year end 2024. The trial is planned to enroll 64 patients that have failed at least one prior therapy across four different 28-day dosing regimens of soquelitinib compared to a placebo group. The endpoints include safety and improvement in Eczema Area and Severity Index ("EASI"). Patients and physicians will be blinded to treatment assignment.
- In February 2024, Corvus presented preclinical data for soquelitinib at the Keystone Symposia on Systemic Autoimmunity and Autoinflammatory Diseases. The data included the first description of Corvus' next-generation ITK inhibitor preclinical product candidates, which were designed to deliver precise T-cell modulation that is optimized for specific immunology indications. These preclinical product candidates exhibit specific biologic properties that are anticipated to enable more precise inhibition of Th1, Th2 and/or Th17 cell function. Atopic dermatitis and asthma are thought to be mediated primarily by Th2 lymphocytes. Th17 cells are associated with psoriasis and psoriatic arthritis. The results suggest that chemical structures may be refined to perform more specific biologic functions and may enable targeting of various types of immune disease. This data builds on the publication of preclinical data on soquelitinib as a preprint at bioRxiv in November 2023 that demonstrated ITK's selective inhibition which produced therapeutic benefits in several autoimmune and allergy preclinical models including psoriasis, asthma, pulmonary fibrosis, scleroderma and graft versus host disease.
- In February 2024, the Company appointed Jeffrey Arcara as Chief Business Officer, with responsibility for leading corporate strategy, business development, portfolio strategy, and new product planning. This includes an initial focus on maximizing the potential of the Company's ITK inhibitor programs, both internally and through partnerships. Mr. Arcara previously served as senior vice president, head global marketing & portfolio and strategy for the innovative medicines and biosimilars business at Teva Pharmaceuticals.
Partner Led Programs: Ciforadenant (adenosine 2a receptor inhibitor) and Mupadolimab (anti-CD73)
- The Kidney Cancer Research Consortium is enrolling a Phase 1b/2 clinical trial evaluating ciforadenant as a potential first line therapy for metastatic renal cell cancer (RCC) in combination with ipilimumab (anti-CTLA-4) and nivolumab (anti-PD-1). The Phase 1b portion of this trial has been completed and patients are now being enrolled in the Phase 2 portion. The clinical trial is expected to enroll up to 60 patients and initial data is anticipated in the first half of 2024.
- Angel Pharmaceuticals, Corvus' partner in China, is enrolling patients in a Phase 1/1b clinical trial of mupadolimab in patients with non-small cell lung cancer (NSCLC) and head and neck squamous cell cancers (HNSCC). In this clinical trial, patients will receive mupadolimab monotherapy or in combination with pembrolizumab.
Financial Results
As of December 31, 2023, Corvus had cash, cash equivalents and marketable securities of $27.1 million as compared to $42.3 million as of December 31, 2022. During the year ending December 31, 2023, the Company sold 2,461,903 shares of its common stock through its at-the-market program, generating net proceeds to the Company of approximately $7.8 million.
Research and development expenses for the three months and full year ended December 31, 2023 totaled $4.0 million and $16.5 million, respectively, compared to $4.1 million and $24.5 million for the same periods in 2022. For the full year 2023, the decrease of $8.0 million was primarily due to lower clinical trial and manufacturing costs associated with the development of mupadolimab.
The net loss for the three months ended December 31, 2023 was $6.7 million compared to a net loss of $9.8 million for the same period in 2022. Total stock compensation expense for the three months ended December 31, 2023 and 2022 was $0.6 million and the non-cash loss from Corvus' equity method investment in Angel Pharmaceuticals was $1.4 million for the three months ended December 31, 2023 compared to $4.6 million in the same period in 2022.
Conference Call Details
Corvus will host a conference call and webcast today, Tuesday, March 19, 2024, at 4:30 p.m. ET (1:30 p.m. PT), during which time management will provide a business update and discuss the fourth quarter and full year 2023 financial results. The conference call can be accessed by dialing 1-877-407-0784 (toll-free domestic) or 1-201-689-8560 (international) or by clicking on this link for instant telephone access to the event. The live webcast may be accessed via the investor relations section of the Corvus website. A replay of the webcast will be available on Corvus' website for 90 days.
About Corvus Pharmaceuticals
Corvus Pharmaceuticals is a clinical-stage biopharmaceutical company pioneering the development of ITK inhibition as a new approach to immunotherapy for a broad range of cancer and immune diseases. The Company's lead product candidate is soquelitinib, an investigational, oral, small molecule drug that selectively inhibits ITK. Its other clinical-stage candidates are being developed for a variety of cancer indications. For more information, visit www.corvuspharma.com.
About Soquelitinib
Soquelitinib (formerly CPI-818) is an investigational small molecule drug given orally designed to selectively inhibit ITK (interleukin-2-inducible T cell kinase), an enzyme that is expressed predominantly in T cells and plays a role in T cell and natural killer (NK) cell immune function. The immunologic effects of soquelitinib lead to what is known as Th1 skewing and is made possible by the high selectivity of soquelitinib for ITK. Research on soquelitinib's mechanism of action suggests that it has the potential to control differentiation of normal T helper cells and enhance immune responses to tumors by augmenting the generation of cytotoxic killer T cells and the production of cytokines that inhibit cancer cell survival. Soquelitinib has also been shown to prevent T cell exhaustion, a major limitation of current immunotherapy and CAR-T therapies. Optimal doses of soquelitinib have been shown to affect T cell differentiation and induce the generation of Th1 helper cells while blocking the development of both Th2 and Th17 cells and production of their secreted cytokines. Th1 T cells are required for immunity to tumors, viral infections and other infectious diseases. Th2 and Th17 helper T cells are involved in the pathogenesis of many autoimmune and allergic diseases. The Company believes the inhibition of specific molecular targets in T cells may be of therapeutic benefit for patients with cancers, including solid tumors, and in patients with autoimmune and allergic diseases.
About Peripheral T Cell Lymphoma
Peripheral T cell lymphoma is a heterogeneous group of malignancies accounting for about 10% of non-Hodgkin's lymphomas (NHL) in Western populations, reaching 20% to 25% of NHL in some parts of Asia and South America. The most common subtypes are PTCL-not otherwise specified (PTCL-NOS) and T follicular helper cell lymphoma. First line treatment for these diseases is typically combination chemotherapy, however, approximately 75% of patients either do not respond or relapse within the first two years. Patients in relapse are treated with various chemotherapy agents but have poor overall outcomes with median progression-free survival in the three to four month range and overall median survival of six to 12 months. There are no approved drugs in relapsed PTCL based on randomized trials.
PTCL is a disease of mature helper T cells that express ITK, often containing numerous genetic mutations and frequently associated with viral infection. Most often the malignant cells of PTCL express a Th2 phenotype.
About Ciforadenant
Ciforadenant (CPI-444) is an investigational small molecule, oral, checkpoint inhibitor designed to disable a tumor's ability to subvert attack by the immune system by blocking the binding of adenosine to immune cells present in the tumor microenvironment. Adenosine, a metabolite of ATP (adenosine tri-phosphate), is produced within the tumor microenvironment where it may bind to the adenosine A2A receptor present on immune cells and block their activity. Ciforadenant has been shown to block the immunosuppressive effects of myeloid cells present in tumors and preclinical studies published in 2018 demonstrated synergy with combinations of anti PD1 and anti-CTLA4 antibodies.
About Mupadolimab
Mupadolimab (CPI-006) is an investigational, potent humanized monoclonal antibody that is designed to react with a specific site on CD73. In preclinical studies, it has demonstrated immunomodulatory activity resulting in activation of lymphocytes, induction of antibody production from B cells and effects on lymphocyte trafficking. While there are other anti-CD73 antibodies and small molecules in development for treatment of cancer, such agents react with a different region of CD73. Mupadolimab is designed to react with a region of the molecule that acts to stimulate B cells and block production of immunosuppressive adenosine. Mupadolimab is being studied in combination with pembrolizumab in a Phase 1b/2 clinical trial in patients with advanced head and neck cancers and in patients with NSCLC that have failed chemotherapy and anti-PD(L)1 therapy. It is postulated that the activation of B cells will enhance immunity within the tumors of these patients, leading to improved clinical outcomes.
About Angel Pharmaceuticals
Angel Pharmaceuticals is a privately held biopharmaceutical company developing a pipeline of precisely targeted investigational medicines for cancer, autoimmune, infectious and other serious diseases in China. Angel Pharmaceuticals was launched through a collaboration with U.S.-based Corvus and investments from investors in China. Angel Pharmaceuticals licensed the rights to develop and commercialize Corvus' three clinical-stage candidates - soquelitinib, ciforadenant and mupadolimab - in greater China and obtained global rights to Corvus' BTK inhibitor preclinical programs. Under the collaboration, Corvus currently has a 49.7% equity stake in Angel Pharmaceuticals excluding 7% of Angel's equity reserved for issuance under the Angel ESOP, and Corvus has designated three individuals on Angel's five-person Board of Directors. For more information, visit www.angelpharma.com.
Forward-Looking Statements
This press release contains forward-looking statements, including statements related to the potential safety and efficacy of the Company's product candidates including soquelitinib, ciforadenant and mupadolimab; the potential use of soquelitinib to treat a variety of hematological cancers and autoimmune diseases; the Company's ability and its partners' ability, as well as the timing thereof, to develop and advance product candidates into and successfully complete preclinical studies and clinical trials, including the Company's Phase 1/1b clinical trial of soquelitinib and its Phase 1b/2 clinical trial of ciforadenant; the timing of and the Company's ability to launch clinical trials, including the potentially registrational Phase 3 clinical trial for soquelitinib; the design of clinical trials, including the timeline for initiation, target number of patients to be enrolled and certain other product development milestones; the availability and timing of clinical data announcements, including initial data from the Phase 1b/2 clinical trial with ciforadenant. All statements other than statements of historical fact contained in this press release are forward-looking statements. These statements often include words such as "believe," "expect," "anticipate," "intend," "plan," "estimate," "seek," "will," "may" or similar expressions. Forward-looking statements are subject to a number of risks and uncertainties, many of which involve factors or circumstances that are beyond the Company's control. The Company's actual results could differ materially from those stated or implied in forward-looking statements due to a number of factors, including but not limited to, risks detailed in the Company's Annual Report on Form 10-K for the year ended December 31, 2023, filed with the Securities and Exchange Commission on or about the date hereof, as well as other documents that may be filed by the Company from time to time with the Securities and Exchange Commission. In particular, the following factors, among others, could cause results to differ materially from those expressed or implied by such forward-looking statements: the Company's ability to demonstrate sufficient evidence of efficacy and safety in its clinical trials of soquelitinib and its other product candidates; the accuracy of the Company's estimates relating to its ability to initiate and/or complete preclinical studies and clinical trials and release data from such studies and clinical trials; the results of preclinical studies and interim data from clinical trials not being predictive of future results; the Company's ability to enroll sufficient numbers of patients in its clinical trials; the unpredictability of the regulatory process; regulatory developments in the United States, and other foreign countries; the costs of clinical trials may exceed expectations; and the Company's ability to raise additional capital. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, it cannot guarantee that the events and circumstances reflected in the forward-looking statements will be achieved or occur, and the timing of events and circumstances and actual results could differ materially from those projected in the forward-looking statements. Accordingly, you should not place undue reliance on these forward-looking statements. All such statements speak only as of the date made, and the Company undertakes no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise. The Company's results for the quarter ended December 31, 2023 are not necessarily indicative of its operating results for any future periods.
| CORVUS PHARMACEUTICALS, INC. CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS (in thousands, except share and per share data) | |||||||||||||||
| Three Months Ended December 31, | Year Ended December 31, | ||||||||||||||
| 2023 | 2022 | 2023 | 2022 | ||||||||||||
| (unaudited) | |||||||||||||||
| Operating expenses: | |||||||||||||||
| Research and development | $ | 3,999 | $ | 4,080 | $ | 16,526 | $ | 24,468 | |||||||
| General and administrative | 1,652 | 1,586 | 6,881 | 8,097 | |||||||||||
| Total operating expenses | 5,651 | 5,666 | 23,407 | 32,565 | |||||||||||
| Loss from operations | (5,651 | ) | (5,666 | ) | (23,407 | ) | (32,565 | ) | |||||||
| Interest income and other expense, net | 380 | 318 | 1,584 | 654 | |||||||||||
| Gain from sale of property and equipment | - | 22 | - | 22 | |||||||||||
| Sublease income - related party | 22 | 148 | 78 | 587 | |||||||||||
| Loss from equity method investment | (1,404 | ) | (4,638 | ) | (5,284 | ) | (10,005 | ) | |||||||
| Net loss | $ | (6,653 | ) | $ | (9,816 | ) | $ | (27,029 | ) | $ | (41,307 | ) | |||
| Net loss per share, basic and diluted | $ | (0.14 | ) | $ | (0.21 | ) | $ | (0.56 | ) | $ | (0.89 | ) | |||
| Shares used to compute net loss per share, basic and diluted | 49,038,582 | 46,553,511 | 48,025,274 | 46,553,511 | |||||||||||
| CORVUS PHARMACEUTICALS, INC. CONDENSED CONSOLIDATED BALANCE SHEETS (in thousands) | ||||||||
| Year ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Assets | ||||||||
| Cash, cash equivalents and marketable securities | $ | 27,149 | $ | 42,303 | ||||
| Operating lease right-of-use asset | 1,149 | 2,217 | ||||||
| Other assets | 1,132 | 1,843 | ||||||
| Investment in Angel Pharmaceuticals | 16,123 | 21,877 | ||||||
| Total assets | $ | 45,553 | $ | 68,240 | ||||
| Liabilities and stockholders' equity | ||||||||
| Accounts payable and accrued liabilities and other liabilities | $ | 5,495 | $ | 9,524 | ||||
| Operating lease liability | 1,374 | 2,601 | ||||||
| Stockholders' equity | 38,684 | 56,115 | ||||||
| Total liabilities and stockholders' equity | $ | 45,553 | $ | 68,240 | ||||
INVESTOR CONTACT:
Leiv Lea
Chief Financial Officer
Corvus Pharmaceuticals, Inc.
+1-650-900-4522
llea@corvuspharma.com
MEDIA CONTACT:
Sheryl Seapy
Real Chemistry
+1-949-903-4750
sseapy@realchemistry.com
Graphs accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/24e399bd-609e-4269-9569-606fc30e1807
https://www.globenewswire.com/NewsRoom/AttachmentNg/238df82b-3dd6-42e0-ad10-bcd225edfede

