
The EU Medical Device Regulation CE certification approves that the most common types of skin cancer and a wide range of skin diseases and conditions can be treated in less than 90 seconds.
COPENHAGEN, Denmark, May 30, 2024 /PRNewswire/ -- TOOsonix A/S, a pioneer in high-intensity focused ultrasound (HIFU) technology for dermatology, has received EU Medical Device Regulation (MDR) CE certification for its system for image-guided dermatologic therapy.

The certification allows TOOsonix to sell its flagship product, System ONE-M, across the European Economic Area. Treatment of skin cancer and skin diseases can now be conducted in ways never before possible, providing more patient-friendly treatment than traditional procedures.
"Our device presents oncologists and dermatologists with a new and versatile tool that can remove the need for an array of lasers, RF-devices, and other expensive specialized tools. With System ONE-M, a typical session may encompass the primary treatment of e.g. cancerous basal cell carcinoma, pre-cancerous actinic keratosis, as well as the removal of various more benign skin conditions that may have emerged since the patient's last visit", said Managing Director at TOOsonix, Torsten Bove.
High precision image-guided therapy
Unlike conventional dermatological therapy, everything can now be accomplished within a single straightforward session using the same device. The clinician can assess the target lesion area in microscopic resolution before treatment using the system's integrated imaging capability, and proceed directly to an image-guided treatment with extreme accuracy.
"The treatment allows patients to depart virtually unaffected and without requiring downtime. Our objective has been to provide the most patient friendly treatment. TOOsonix System ONE-M is the next level device for treatment of the skin", said Co-Managing Director, Tomasz Zawada at TOOsonix.
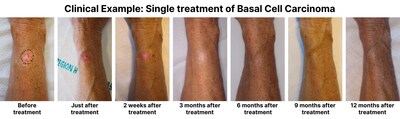
The approved treatments span several subcategories within dermatology. In oncology, interventions cover the treatment of basal cell carcinoma, the most prevalent cancer worldwide, and actinic keratosis, the most common pre-cancerous condition. Additionally, the system offers a pioneering non-invasive treatment of cutaneous neurofibromas in patients with the rare disease Neurofibromatosis Type 1, the most widespread genetic disorder. Finally, a wide range of benign skin tumors and neoplasms within general dermatology are approved. Treatments of these encompass several tens of millions conducted in hospitals and private practice every year, utilizing lasers, light therapies, RF-therapy, cryotherapy, and a range of topical and systemic pharmaceutical products.
Immense potential
Clinical data for all treatments demonstrate safe, swift, and efficient therapies, now providing patients with a non-invasive option that have minimal discomfort and no downtime following treatments, and no side effects from pharmaceutical agents or harmful radiation. In many cases, a single treatment session taking less than 90 seconds suffices to achieve the desired therapeutic outcome.
Lone Schøtt Kunøe, CEO of Consolidated Holdings A/S and Chairman of TOOsonix, remarks:
"Introducing a safe, swift, and effective system to the market for both medical and aesthetic dermatological therapy holds immense potential. I view the CE mark as the initial realization of TOOsonix's breakthrough in the market, and I am tremendously enthusiastic about the excellent prospects for the future".
About TOOsonix A/S
At TOOsonix, our core passion and mission revolve around aiding people through focused ultrasound technology. Collaborating with clinicians and healthcare experts across Europe and the USA, we develop improved clinical options for dermatological therapy. Our clinically approved treatments address some of the most prevalent cancerous, pre-cancerous, genetic, and benign skin conditions of our era. Through our efforts, we aspire to elevate standards of dermatological care and create a healthier future for the patients of today and tomorrow.
More information: www.toosonix.com
Contact
Torsten Bove
Managing Director
Ph: +45 2059 2999
Email: torsten.bove@toosonix.com
Photo - https://mma.prnewswire.com/media/2425801/TOOsonix_A_S.jpg
Photo - https://mma.prnewswire.com/media/2425802/TOOsonix_A_S_2.jpg
Photo - https://mma.prnewswire.com/media/2425803/TOOSonix_A_S_3.jpg
Logo - https://mma.prnewswire.com/media/2425800/TOOsonix_Logo.jpg


![]() View original content:https://www.prnewswire.co.uk/news-releases/toosonix-receives-mdr-ce-mark-for-its-new-oncology-and-dermatology-device-302159356.html
View original content:https://www.prnewswire.co.uk/news-releases/toosonix-receives-mdr-ce-mark-for-its-new-oncology-and-dermatology-device-302159356.html


