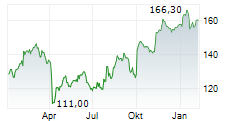
LONDON (dpa-AFX) - AstraZeneca Plc. (AZN.L) said that results from the ECHO Phase III trial showed the company's Calquence (acalabrutinib) in combination with bendamustine and rituximab demonstrated a statistically significant and clinically meaningful improvement in progression-free survival (PFS) and showed a favourable trend in overall survival or OS compared to standard-of-care chemoimmunotherapy (bendamustine plus rituximab) in previously untreated patients with mantle cell lymphoma or MCL.
Results showed the Calquence combination regimen reduced the risk of disease progression or death by 27% compared to standard-of-care chemoimmunotherapy. Median PFS was 66.4 months for patients treated with the Calquence combination versus 49.6 months with standard-of-care chemoimmunotherapy.
The secondary endpoint of overall survival showed a favourable trend for the Calquence combination compared to standard-of-care chemoimmunotherapy, further supporting the clinical benefit of this combination . The overall survival data were not mature at the time of the analysis and the trial will continue to assess overall survival as a key secondary endpoint.
In addition, Results from the ongoing Phase I, dose-escalation trial of AZD0486, a novel CD19xCD3 T cell engager, showed durable responses in patients with heavily pretreated relapsed/refractory follicular lymphoma with a median follow up of 11 months. Complete response rates of 84% were seen at doses of AZD0486 of 2.4 mg and above. Data also showed how cytokine release syndrome (CRS) events were effectively mitigated by the double step-up dosing schedule and no immune effector cell-associated neurotoxicity syndrome (ICANS) events were observed.
For More Such Health News, visit rttnews.com
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News