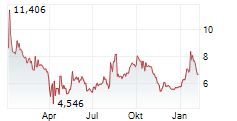
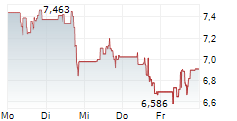
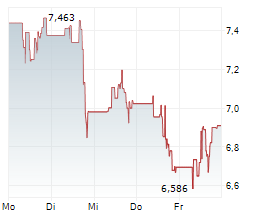
BRUSSELS/FRANKFURT/PARIS (dpa-AFX) - Novavax (NVAX) has filed for a type II variation of existing Marketing Authorization with the European Medicines Agency for JN.1 COVID-19 vaccine, NVX-CoV2705, for individuals aged 12 and older. The company said the submission is in line with guidance from EMA and the World Health Organization to target the JN.1 lineage this fall.
John Jacobs, President and Chief Executive Officer, Novavax, said: 'Our updated COVID-19 vaccine is active against current circulating strains, including KP.2 and KP.3.'
Novavax plans to have its vaccine in unit-dose vials available for distribution in the European Union for immediate release post-approval.
For More Such Health News, visit rttnews.com.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News