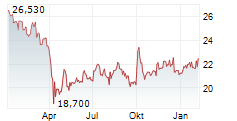
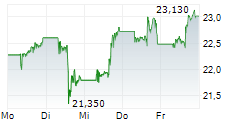
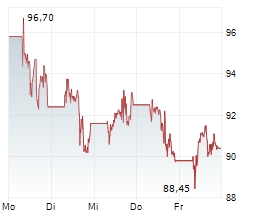
NEW YORK CITY (dpa-AFX) - Pfizer Inc. (PFE) and BioNTech SE (BNTX) Thursday announced that the Committee for Medicinal Products for Human Use or CHMP of the European Medicines Agency has recommended marketing authorization for its Omicron JN.1-adapted monovalent COVID-19 vaccine for active immunization.
This drug is intended to prevent COVID-19 caused by SARS-CoV-2 in individuals 6 months of age and older. The adaptation is based on the World Health Organization's Technical Advisory Group on COVID-19 Vaccine Composition and the European Medicines Agency's Emergency Task Force.
The European Commission will review the CHMP's recommendation and will make a final decision.
The CHMP's recommendation is based on the full body of previous clinical, non-clinical, and real-world evidence supporting the safety and efficacy of the COVID-19 vaccines by Pfizer and BioNTech.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News