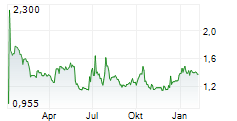
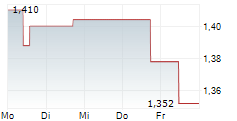
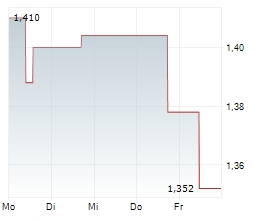
BRUSSELS/FRANKFURT/PARIS (dpa-AFX) - AB Science SA (ABSCF.PK) said the Committee for Medicinal Products for Human Use of the European Medicines Agency has adopted a negative opinion on the application for conditional marketing authorization of masitinib in the treatment of amyotrophic lateral sclerosis. AB Science plans to ask for re-examination. The company said the CHMP confirmed that the safety of masitinib is deemed acceptable, which is a key consideration in the context of a conditional marketing authorization where confirmatory evidence of efficacy is required.
AB Science also highlighted the difficulty of a conditional marketing authorization in ALS and said it cannot guarantee a positive outcome following the re-examination.
For More Such Health News, visit rttnews.com.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News