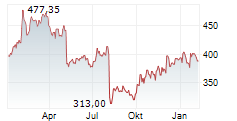
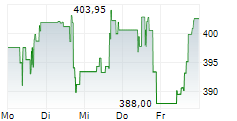
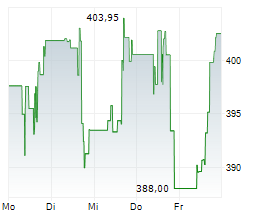
CAMBRIDGE (MASSACHUSETTS) (dpa-AFX) - Vertex Pharmaceuticals (VRTX) announced Health Canada has granted Marketing Authorization for the expanded use of TRIKAFTA for the treatment of cystic fibrosis in patients aged 2 years and older who have a mutation in the cystic fibrosis transmembrane conductance regulator gene that is responsive based on clinical and/or in vitro data. TRIKAFTA was previously approved for use in people with CF aged 2 years and older with at least one F508del mutation and is now approved for 152 additional mutations.
The company noted that the label update is based on data from multiple sources, including a 24-week randomized placebo-controlled double-blind study in patients aged 6 years and older who had at least one qualifying non-F508del mutation to evaluate the safety and efficacy of TRIKAFTA.
For More Such Health News, visit rttnews.com.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News