NORTHAMPTON, MA / ACCESSWIRE / July 18, 2024 / Viatris is a global healthcare company focused on bringing high-quality medicines to patients through a hybrid approach that bridges the traditional divide between generics and brands, combining the best of both, to more holistically address healthcare needs globally. The diversity of our portfolio, global footprint and our mission to empower people worldwide to live healthier at every stage of life are our foundational strengths.
Our business and operating model is deliberately designed and implemented to deliver on our strategy to build and sustain access to medicine at scale. Underpinned by Viatris' relevance and success in meeting evolving healthcare needs, we seek to create value for and together with our key stakeholders - the people who trust our medicines every day, the health systems who rely on us, the people who make up Viatris, our partners and the investors who believe in our ability to execute on our ambitious mission.
We are convinced that patients and health systems around the world are best served by a healthcare company applying a well-rounded and longterm approach, maintaining viability while working to manage inherent risks and opportunities, and continuously striving to advance sustainable operations and responsible practices in a focused way.
Our Commitment to Access
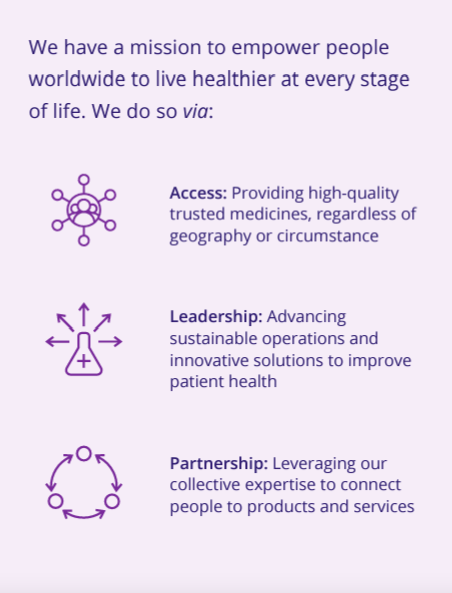
Access is fundamental to our mission. It is not an initiative; it is our business model. It begins with our ability to sustainably deliver quality medicines at scale to people, regardless of geography or circumstance. With an extensive portfolio of medicines to address nearly every health need, a one-of-a-kind global supply chain designed to reach more people with health solutions when and where they need them, and the scientific expertise to address some of the world's most enduring health challenges, access is central to everything we do.
From our unique vantage point, we touch all of life's moments, from birth to the end of life, acute conditions to chronic diseases. We see across multiple therapeutic areas to the person at the center of their own unique health journey. We are focused on meeting individual needs, whether with a generic medicine, a trusted brand, an improved version of an existing medicine, or a truly novel therapeutic solution.
We go beyond developing, making and distributing high-quality medicines and work to help find solutions that support resilient systems for health. We have designed our global operations and supply chain to be a reliable and flexible partner to enable access to medicines across the world, constantly adapting to an ever-evolving and increasingly dynamic landscape.
Partnerships and collaborations are critical for meaningful and lasting impact, as are policies and strong healthcare systems that allow for healthy competitive environments. While needs are universal, circumstances are local, and we work with an array of organizations - globally, regionally and locally, public and private - to support sustainable access to medicines at consistent quality standards. We work to connect more people with even more products and services to advance access and health. Ultimately, we know we are stronger together, working collaboratively and relentlessly across our company and with the broader global community, in pursuit of access.
Providing a Sustainable, Diverse and Differentiated Portfolio
In 2023, Viatris sold more than 80 billion doses of medicine across more than 165 countries and territories, reaching more than 90% of LMICs. We supply medicines for patients across a broad range of major therapeutic areas. Viatris offers quality treatment options across more than 10 major therapeutic areas covering a wide variety of NCDs and infectious diseases. We also offer support services such as diagnostic clinics, educational seminars and digital tools to help patients better manage their health. We offer a broad and diverse range of product options across all our therapeutic areas, with many categories containing several products in a range of dosage forms, formulations and delivery systems that allow physicians to tailor care for people's needs.
Meeting Unmet Needs With Added Competencies
Viatris has announced a well-established strategic vision to build for a future where we remain a relevant partner in meeting unmet needs and supporting healthcare systems across the globe, in an ever-evolving landscape. In 2023, we worked diligently to build a strong foundation for executing on the next phase of our strategic plan, including making significant progress on our previously announced divestitures. More information about the divestitures is provided on page 89.
We are not changing who we are; we are focusing on our core competencies and adding to our capabilities to better meet unmet needs. We intend to continue building on our strong existing access-driven base business while diligently pursuing important new opportunities, with a clear focus on limited-competition complex generics and novel products targeting gaps in care, all with a first-to-market emphasis where our scientific and development expertise can help further accelerate access. In addition to novel products, we will continue to focus on making improvements to existing molecules, including new formulations, to better meet the needs of people worldwide. Complex product categories are critical to patient health and are growing at a rapid pace. Our goal is to enhance our proven scientific capabilities and current global platform, ultimately seeking opportunities to further advance reliable access to medicine and unlock shareholder value.
We regularly review the products we currently provide across different markets, which may periodically lead to expanded registration of products with unmet need or rationalization of products that are no longer viable or in demand. Throughout this process, we work to carefully consider the availability of alternatives for patients to avoid disruption of critical medications.
Deep In-House Development Capabilities and a Diverse Pipeline
We expect to expand further into development of more innovative molecules, including new chemical entities (NCEs) and improved versions of existing products, such as those filed through the U.S. FDA's 505(b)(2) pathway.
We have a strong pipeline across eye care, complex injectables and novel products and more than 98% of expected new product launches by 2023 are either launched, approved or pending approval. During the past year, we integrated Oyster Point and Famy Life Sciences acquisitions and successfully created our Eye Care Division. Over 2023, we further advanced our pipeline across complex injectables, novel and complex products. We have a proven track record of delivering industry firsts that have enabled us to address some of the world's most enduring health challenges.
In 2023, we advanced many new products addressing diverse needs, spanning generic medicines, improvements to existing molecules and novel products. We launched Breyna (budesonide and formoterol fumarate dihydrate) Inhalation Aerosol, the first U.S. FDA-approved generic version of Symbicort® to use for certain patients with asthma or chronic obstructive pulmonary disease (COPD). We received positive top-line phase 3 trial results for Yupelri® (revefenacin) in China, an important step in advancing access to this treatment for COPD. The U.S. FDA approved RYZUMVI1 (phentolamine ophthalmic solution) 0.75% eye drops for the treatment of pharmacologically induced mydriasis. We also received U.S. FDA tentative approval of a strawberry-flavored, single tablet regimen for oral suspension - the fixed-dose combination of abacavir, dolutegravir and lamivudine - that will reduce the pill burden for children living with HIV across low- and middle-income countries.
In 2024, we acquired the development programs and certain personnel related to Selatogrel and Cenerimod from Idorsia, a Swiss biotech company. This collaboration is an important step in expanding our portfolio of innovative assets with great potential to help bridge gaps in treatment. The products in development are Selatogrel, under investigation for the treatment of heart attacks, and Cenerimod, under investigation for the treatment of systemic lupus erythematosus (SLE).
In line with our mission, we look forward to leveraging our proven track record to pursue opportunities across our diverse portfolio and pipeline to bring access to new and improved treatments for the benefit of people worldwide.
View the full 2023 Sustainability Report.
Sources
1 Viatris announced the launch of the product on April 1, 2024
View additional multimedia and more ESG storytelling from Viatris on 3blmedia.com.
Contact Info:
Spokesperson: Viatris
Website: https://www.3blmedia.com/profiles/viatris
Email: info@3blmedia.com
SOURCE: Viatris
View the original press release on accesswire.com
