LONDON (dpa-AFX) - SCYNEXIS Inc. (SCYX) announced it will receive a $10 million development milestone payment from GSK under their license agreement for ibrexafungerp. The milestone payment is triggered by the delivery of final clinical study reports for the completed FURI, CARES, and NATURE clinical studies.
The company noted that results from the open-label studies of ibrexafungerp in patients with refractory or resistant fungal diseases (FURI) and in patients with Candidiasis caused by Candida auris (CARES) are positive and consistent with previously announced interim analyses and are expected to be presented at a future scientific meeting. NATURE was an observational study to evaluate the outcome of patients with invasive candidiasis treated with available standard of care options and was designed as an external control strategy for the FURI study.
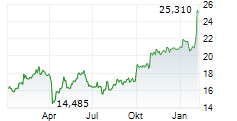
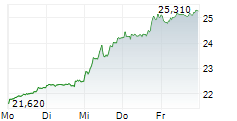
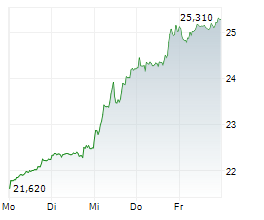
SCYNEXIS anticipates receiving the $10 million milestone payment in the third quarter of 2024.
As per the terms of the exclusive license agreement with GSK, SCYNEXIS had previously received $115 million in upfront and development milestone payments and is eligible to receive up to an additional $323 million in potential development, regulatory and commercial milestone-based payments for a total deal value of $448 million. GSK will also pay mid-single digit to mid-teen digit tiered royalties on the totality of ibrexafungerp sales across all indications.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News