WASHINGTON (dpa-AFX) - Actinium Pharmaceuticals (ATNM) issued a regulatory update on the planned Biologics License Application filing for Iomab-B in patients with active relapsed or refractory acute myeloid leukemia. The company has concluded both clinical and Chemistry, Manufacturing and Controls interactions with the FDA regarding the BLA pathway for Iomab-B. The FDA has now determined that the analyses from the SIERRA trial do not adequately support a BLA filing for Iomab-B. The FDA has now determined that demonstrating an overall survival benefit in a randomized head-to-head trial is required for a BLA filing.
The FDA has advised Actinium to conduct a study to evaluate allogeneic bone marrow transplant using Iomab-B plus a reduced intensity conditioning regimen of fludarabine and total body irradiation versus allogeneic BMT using reduced intensity conditioning comprised of cyclophosphamide plus Flu/TBI, a difference from the SIERRA trial. Also, the proposed additional trial will not allow crossover, which was allowed in SIERRA.
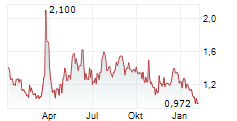
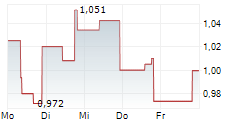
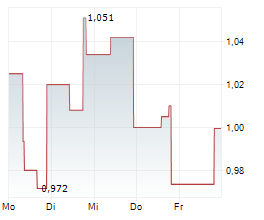
Shares of Actinium Pharmaceuticals are down 64% in pre-market trade on Monday.
For More Such Health News, visit rttnews.com.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News