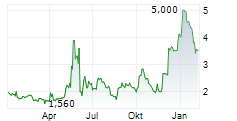
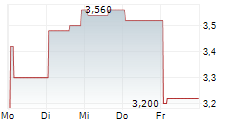
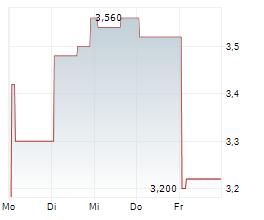
Vancouver, British Columbia--(Newsfile Corp. - August 6, 2024) - NervGen Pharma Corp. (TSXV: NGEN) (OTCQB: NGENF), a clinical-stage biotech company dedicated to developing innovative solutions for the treatment of nervous system damage, announced today that Mr. Mike Kelly, President & CEO, will be presenting at the Canaccord Genuity 44th Annual Growth Conference, on August 13, 2024, at 10:00 a.m. EDT and members of management will be available for one-on-one investor meetings at the conference.
The conference is being held in-person August 13-15, 2024, at the InterContinental Boston Hotel in Boston, MA. A webcast of the presentation will be available here and posted for replay following the event.
About NervGen
NervGen (TSXV: NGEN, OTCQB: NGENF) is a clinical-stage biotech company dedicated to developing innovative treatments to enable nervous system repair in the settings of traumatic injury and disease. NervGen's lead drug candidate, NVG-291, is being evaluated in a Phase 1b/2a clinical trial in the company's initial target indication, spinal cord injury. The company has initiated preclinical evaluation of a new development candidate, NVG-300, in models of ischemic stroke, amyotrophic lateral sclerosis (ALS) and spinal cord injury. For more information, visit www.nervgen.com and follow NervGen on X, LinkedIn, and Facebook for the latest news on the company.
About NVG-291
NervGen holds exclusive worldwide rights to NVG-291, a first-in-class therapeutic peptide targeting mechanisms that interfere with nervous system repair. NVG-291 is derived from the intracellular wedge domain of the receptor type protein tyrosine phosphatase sigma (protein tyrosine phosphatase sigma). NVG-291-R, a rodent analog of NVG-291, has been shown to promote nervous system repair and functional recovery in animal models of spinal cord injury (acute and chronic intervention), peripheral nerve injury, multiple sclerosis and stroke, through enhanced plasticity, axonal regeneration, and remyelination. NVG-291 has received Fast Track designation in spinal cord injury from the U.S. Food and Drug Administration.
Contacts
Huitt Tracey, Corporate Communications
htracey@nervgen.com
604.537.2094
Bill Adams, Chief Financial Officer
info@nervgen.com
778.731.1711
David Schull or Ignacio Guerrero-Ros, Ph.D.
Russo Partners
david.schull@russopartnersllc.com
ignacio.guerrero-ros@russopartnersllc.com
858.717.2310
646.942.5604
Neither the TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Cautionary Note Regarding Forward-Looking Statements
This news release may contain "forward-looking information" and "forward-looking statements" within the meaning of applicable Canadian and United States securities legislation. Such forward-looking statements and information herein include, but are not limited to, the Company's current and future plans, expectations and intentions, results, levels of activity, performance, goals or achievements, or any other future events or developments constitute forward-looking statements, and the words "may", "will", "would", "should", "could", "expect", "plan", "intend", "trend", "indication", "anticipate", "believe", "estimate", "predict", "likely" or "potential", or the negative or other variations of these words or other comparable words or phrases, are intended to identify forward-looking statements. Forward-looking statements include, without limitation, statements relating to: the subject matter to be presented at the upcoming investor conference; the objectives, timing and study design of the clinical development of NVG-291 in spinal cord injury; the development plans and prospective target indications for NVG-300; and the creation of innovative treatments of nervous system damage due to trauma or disease.
Forward-looking statements are based on estimates and assumptions made by the Company in light of management's experience and perception of historical trends, current conditions and expected future developments, as well as other factors that we believe are appropriate and reasonable in the circumstances. In making forward-looking statements, the Company has relied on various assumptions, including, but not limited to: the Company's ability to manage the effects of pandemics such as COVID-19; the accuracy of the Company's financial projections; the Company obtaining positive results in its clinical and other trials; the Company obtaining necessary regulatory approvals; and general business, market and economic conditions.
Many factors could cause our actual results, level of activity, performance or achievements or future events or developments to differ materially from those expressed or implied by the forward-looking statements, including without limitation, a lack of revenue, insufficient funding, the impact of pandemics such as COVID-19, reliance upon key personnel, the uncertainty of the clinical development process, competition, and other factors set forth in the "Risk Factors" section of the Company's Annual Information Form, Prospectus Supplement, financial statements and Management Discussion and Analysis which can be found on SEDARplus.ca. All clinical development plans are subject to additional funding.
Readers should not place undue reliance on forward-looking statements made in this news release. Furthermore, unless otherwise stated, the forward-looking statements contained in this news release are made as of the date of this news release, and we have no intention and undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by applicable law. The forward-looking statements contained in this news release are expressly qualified by this cautionary statement.

To view the source version of this press release, please visit https://www.newsfilecorp.com/release/218987
SOURCE: NervGen Pharma Corp.