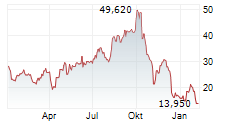
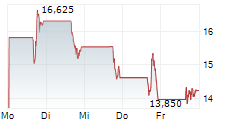
SAN DIEGO, Aug. 12, 2024 (GLOBE NEWSWIRE) -- LENZ Therapeutics, Inc. (Nasdaq: LENZ or "LENZ" or the "Company"), a late clinical-stage biopharmaceutical company focused on developing the first and only aceclidine-based eye drop to improve near vision in people with presbyopia, today announced that the Company has submitted a New Drug Application (NDA) to U.S. Food and Drug Administration (FDA) for LNZ100 (an aceclidine-based ophthalmic solution) for the treatment of presbyopia, a condition impacting an estimated 1.8 billion people globally and 128 million people in the United States.
"The submission of our NDA for LNZ100 is a significant milestone for LENZ and is a testament to the tremendous focus, execution and collaboration of our team," said Eef Schimmelpennink, President and Chief Executive Officer of LENZ Therapeutics. "We believe LNZ100 has the potential to be best-in-class as a pupil-selective and long-acting therapeutic option for the treatment of presbyopia. We look forward to working alongside the FDA through this review process."
The NDA submission is supported by positive data from the pivotal Phase 3 CLARITY study of LNZ100 for the treatment of presbyopia. In the Phase 3 CLARITY study LNZ100 achieved all primary and secondary near vision improvement endpoints with statistically significant three-lines or greater improvement in Best Corrected Distance Visual Acuity (BCDVA) at near, without losing one line or more in distance visual acuity, demonstrating LNZ100 was well tolerated with no serious treatment-related adverse events observed in the over 30,000 treatment days monitored in the CLARITY study.
The FDA has a 60-day filing review period to determine whether the NDA submission is complete and acceptable for review.
About LENZ Therapeutics
LENZ Therapeutics is a late clinical-stage biopharmaceutical company focused on the development and commercialization of the first and only aceclidine-based eye drop to improve vision in patients with presbyopia. LENZ's product candidate, LNZ100 is a preservative-free, single-use, once-daily eye drop containing aceclidine. LNZ100 was evaluated in the registration-enabling Phase 3 CLARITY study as a potential therapy for the treatment of presbyopia, a condition impacting an estimated 1.8 billion people globally and 128 million people in the United States. LENZ is committed to commercializing an ideal pharmaceutical presbyopia solution that enhances vision for "all eyes, all day." LENZ is headquartered in San Diego, California. For more information, visit: LENZ-Tx.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of federal securities laws. You can identify forward-looking statements by words such as "may," "will," "could," "can," "would," "should," "expect," "intend," "plan," "anticipate," "believe," "estimate," "predict," "project," "potential," "poised," "continue," "ongoing" or the negative of these terms or other comparable terminology, but not all forward-looking statements will contain these words. Forward-looking statements in this press release include, but are not limited to, statements regarding the review and potential approval of our NDA by FDA for the potential regulatory approval and commercialization of LNZ100, if approved; the size of the market opportunity for LNZ100; the beneficial characteristics of LNZ100 and its expected impact on presbyopes; and expectations regarding shareholder value creation. These statements are based on numerous assumptions concerning the development of LENZ's product candidates and target markets and involve substantial risks, uncertainties and other factors that may cause actual results, levels of activity, performance or achievement to be materially different from the information expressed or implied by these forward-looking statements, including those risk factors described in the section titled "Risk Factors" in our Form 10-Q filed with the SEC on May 8, 2024, and our subsequent filings with the SEC. We cannot assure you that the forward-looking statements in this press release or the assumptions upon which they are based will prove to be accurate. The forward-looking statements in this press release are as of the date of this press release. Except as otherwise required by applicable law, LENZ disclaims any duty to update any forward-looking statements. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this press release.
Contacts:
Dan Chevallard
LENZ Therapeutics, Inc.
IR@LENZ-Tx.com