NEW BRUNSWICK (dpa-AFX) - Embattled German conglomerate Bayer, which reduced its workforce by 3,200 positions in the first half of the year, has reportedly announced further layoffs this week as it continues to navigate its financial and operational challenges. In a setback for Aadi, the company's plans to widen the use of its key drug were derailed by disappointing results from a phase II solid tumor trial. Meanwhile, on the regulatory front, the FDA has approved updated COVID-19 vaccines that target KP.2 strain to enhance protection this fall. Additionally, Johnson & Johnson made headlines by expanding its MedTech cardiovascular portfolio through a new acquisition, marking a significant move in its growth strategy.
Let's dive into the details.
Layoffs
Aadi Bioscience Inc. (AADI), on Wednesday, announced it is reducing its Research & Development workforce by 80%, following its decision to halt the registration-intended PRECISION1 trial. PRECISION1 is a phase II study of Fyarro (nab-sirolimus) in patients with solid tumors harboring TSC1 or TSC2 inactivating alterations. An analysis by the Independent Data Monitoring Committee demonstrated that the study was unlikely to exceed an efficacy threshold necessary to support an accelerated approval, the key goal of this study. The company expects to extend cash runway into at least 2H 2026. Fyarro, the company's marketed drug, was approved in the U.S. in 2021 for the treatment of malignant perivascular epithelioid cell tumor (PEComa).
AADI closed Friday's trading at $1.58, up 1.94%.
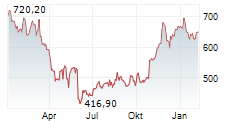
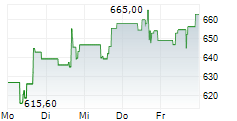
German pharmaceutical and biotech giant Bayer (BAYRY.OB) is cutting around 150 of its 1,000 full-time jobs, mainly in its Consumer Health division in Basel, Switzerland, reported NZZ on Tuesday. The company, which is under significant financial strain, had reduced its workforce by 3,200 in the first half of this year, as part of its restructuring program. The net financial debt totaled €36.76 billion as of June 30, 2024.
BAYRY.OB closed Friday's trading at $7.81, up 0.26%.
FDA Approvals and Rejections
The FDA, on Thursday, approved Pfizer Inc. (PFE) and BioNTech SE's (BNTX) updated COVID-19 vaccine that targets KP.2 strain of the JN.1 lineage for individuals 12 years of age and older. The JN.1 variant of SARS-CoV-2, the virus that causes COVID-19, accounted for approximately 62% of all circulating SARS-CoV-2 variants earlier this year, according to the CDC. The companies' Omicron KP.2-adapted 2024-2025 Formula COVID-19 vaccine was also granted emergency use authorization for individuals 6 months through 11 years of age the same day. Moderna Inc.'s (MRNA) updated COVID-19 vaccine that targets the KP.2 variant has also been approved by the FDA for individuals 12 years of age and older.
PFE closed Friday's trading at $28.90, up 0.42%.
Regeneron Pharmaceuticals Inc. (REGN), on Tuesday, announced that the FDA declined to approve its investigational drug Linvoseltamab, proposed for the treatment of relapsed/refractory multiple myeloma that has progressed after at least three prior therapies. The denial was tied to findings from a pre-approval inspection at a third-party fill/finish manufacturer for another company's product candidate. The third-party fill/finish manufacturer has since informed Regeneron that it believes the issues in the facility have been resolved, and that it is awaiting FDA reinspection, which is expected to take place in the coming months.
REGN closed Friday's trading at $1,199.12, up 0.96%.
Johnson & Johnson's (JNJ) combination of RYBREVANT and LAZCLUZE, on Tuesday, received FDA approval for the first-line chemotherapy-free treatment of locally advanced or metastatic EGFR-mutated non-small-cell lung cancer. The drug in combination with chemotherapy received FDA approval for first-line treatment of patients with non-small cell lung cancer with EGFR Exon 20 insertion mutations as recently as March of this year.
JNJ closed Friday's trading at $164.13, up 1.10%.
Liquidia Corp. (LQDA), on Monday, announced that the FDA has granted tentative approval for Yutrepia inhalation powder to treat adults with pulmonary arterial hypertension and pulmonary hypertension associated with interstitial lung disease. The same day, the U.S. regulatory agency granted a 3-year new clinical investigation exclusivity (NCI exclusivity) to United Therapeutics Corp.'s (UTHR) marketed drug Tyvaso DPI, which is also indicated for the treatment of pulmonary arterial hypertension and pulmonary hypertension associated with interstitial lung disease. The two companies are embroiled in a long-standing patent battle, with United Therapeutics alleging that Liquidia's Yutrepia infringes on Tyvaso patent.
LQDA closed Friday's trading at $9.97, up 3.53%.
Deal or No Deal
Johnson & Johnson (JNJ), on Tuesday, agreed to acquire Israel-based V-Wave, a privately held company that develops innovative treatment options for patients with heart failure, for an upfront payment of $600 million, with the potential for additional regulatory and commercial milestone payments up to approximately $1.1 billion. V-Wave will join Johnson & Johnson as part of Johnson & Johnson MedTech. The transaction is expected to close before the end of 2024. In April of this year, Johnson & Johnson acquired Nasdaq-listed cardiovascular medical device company Shockwave Medical Inc., for approximately $13.1 billion.
JNJ closed Friday's trading at $164.13, up 1.10%.
Dr. Reddy's Laboratories (RDY) and its CRDMO arm Aurigene Pharmaceutical Services Limited, on Tuesday, announced that they are partnering with Kainomyx Inc., a US-based company, for joint development of affordable anti-malarial drug. This potential partnership aims to bring together Kainomyx's expertise in novel drug discovery, Aurigene's capability to conduct integrated drug discovery and development, and Dr. Reddy's ability to commercialize products in low and middle-income countries, in addition to the US and Europe.
RDY closed Friday's trading at $82.16, down 0.54%.
Clinical Trials - Breakthroughs and Setbacks
Jazz Pharmaceuticals plc (JAZZ), on Friday, announced disappointing top-line results from a phase III trial in Japan evaluating the safety and efficacy of cannabidiol oral solution as an adjunctive treatment for seizures associated with Lennox-Gastaut syndrome (LGS), Dravet syndrome (DS) or tuberous sclerosis complex (TSC) in Japanese pediatric patients. Nevertheless, the company will continue to collect data in Japanese patients and plans to engage with regulatory authorities in Japan regarding a potential new drug application. The cannabidiol oral solution is approved and marketed under the tradename Epidiolex for the treatment of seizures associated with LGS, Dravet syndrome or TSC in patients one year of age and older. In the European Union, it is approved under the tradename Epidyolex for adjunctive use in conjunction with clobazam to treat seizures associated with LGS and Dravet syndrome in patients two years and older, and for adjunctive use to treat seizures associated with TSC, in patients two years of age and older. Epidiolex/Epidyolex generated net product sales of $247.1 million in the second quarter of 2024, an increase of 22% compared to the same period in 2023.
JAZZ closed Friday's trading at $113.87, down 0.59%.
Sutro Biopharma Inc. (STRO), on Thursday, announced that it has initiated REFR?ME-L1, the global phase II study of investigational drug Luvelta for patients with non-small cell lung cancer whose tumor expresses Folate Receptor-? (FR?). Initial data from this study is expected in the first half of 2025.
STRO closed Friday's trading at $5.01, up 10.11%.
Nykode Therapeutics ASA (VACBF.PK), on Wednesday, announced its decision to discontinue a phase II trial of its off-the-shelf therapeutic cancer vaccine candidate VB10.16 in second line HPV16-positive cervical cancer, dubbed VB-C-04, as part of its strategic repositioning for VB10.16. The company has decided to focus the development of VB10.16 on locally advanced cervical cancer and recurrent metastatic head and neck cancer. The company expects to reduce VB10.16 development costs by over $25 million and extend its cash runway.
VACBF.PK closed Friday's trading at $0.95, down 24%.
Palatin Technologies Inc. (PTN), on Thursday, announced that its phase II trial evaluating Bremelanotide co-administered with Tirzepatide for the treatment of Obesity has dosed the first subjects. The trial, dubbed BMT-801, is designed to enrol 60 patients and enrolment is expected to be completed this quarter. Topline results from this study are anticipated in the first quarter of 2025.
PTN closed Friday's trading at $1.54, up 4.05%.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News