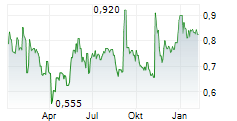
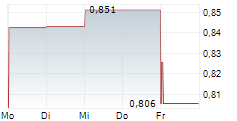
STOCKHOLM, Sept. 13, 2024 /PRNewswire/ -- Moberg Pharma AB (OMX: MOB) announces that the company has received information about clinical cure in a subset of patients in the ongoing North American Phase 3 study for MOB-015 against nail fungus. The number of patients who have achieved clinical cure in this blinded subset of patients is lower than the company's expectations, which necessitates that Moberg Pharma inform the market about this fact.
The North American Phase 3 study is ongoing at 33 study centers in the US and Canada, including a total of 384 patients. The study constitutes an essential part of the clinical data required for the registration and commercialization of MOB-015 in the US and differs from previous studies with MOB-015, which is the basis for drug approval in 13 EU countries, by reducing the dosage - 8 weeks daily dosing followed by weekly maintenance treatment for 40 weeks, compared to daily dosing throughout the entire treatment period.
In the process of preparing the database for upcoming topline data, the company has received information regarding clinical cure in a subset of patients in the study.
Clinical cure is one of three parameters that together constitute the study's primary treatment goal, complete cure. All three parameters; clinical cure, negative fungal culture, and negative microscopy, need to be met for a patient to be considered completely cured. No information has been obtained about the other study parameters included in complete cure.
The information obtained is blinded; no information has been received regarding which patient received active treatment or how many patients in the data subset received active treatment (patients in the study are randomized 2:1 to treatment with MOB-015 and vehicle).
The total number of patients who have achieved clinical cure in this subset of patients is lower than the company's expectations, and Moberg Pharma assesses that the risk of not being able to commercialize the product in the US based on this study has significantly increased, which requires the company to inform the market of this fact.
It is an absolute priority to protect the integrity of the study data, both as not to undermine the possibilities of using study results in discussions with regulatory authorities, and as there are patients with ongoing treatment in the study.
Moberg Pharma will not speculate on possible outcomes or what this means for the future potential of MOB-015 and will await topline results to avoid drawing premature conclusions.
"Our main priority is to protect the data integrity of the study. Together with our CRO, we will do our utmost to minimize the time from the last patient's last visit to top-line data, and our expectation is that these may be brought forward compared to the timelines previously communicated, before year-end" says Anna Ljung, CEO of Moberg Pharma.
On September 13 th, 2024, at 15:00 (CET), Moberg Pharma's CEO Anna Ljung, CMO Anders Bröijersén, and CSO Amir Tavakkol will answer questions during a telephone conference. The Q&A session will be held in English.
To participate in the conference, please dial in on one of the numbers below before the conference starts:
SE: +46 8 10 884 80 16. Access Code: 961943
US: +1 855 979 6654. Access Code: 961943
For additional information, please contact:
Anna Ljung, CEO, telephone: +46 70 766 60 30, e-mail: [email protected]
Anders Bröijersén, Chief Medical Officer, telephone: + 46 76 001 15 76, e- mail: [email protected]
Amir Tavakkol, Chief Scientific Officer, telephone: +1 973 307 4856, e- mail: [email protected]
About this information
This information is information that Moberg Pharma AB (publ) is obliged to make public pursuant to the EU Market Abuse Regulation. The information was submitted for publication at 8.00 am CEST on September 13 th, 2024, through the contact persons above.
About MOB-015 and Onychomycosis
Approximately 10% of the general population suffer from onychomycosis and a majority of those afflicted go untreated. The global market opportunity is significant with more than hundred million patients worldwide and a clear demand for better products. MOB-015 is an in-house developed topical formulation of terbinafine, enabling effective concentrations of terbinafine to the nail and nail bed while avoiding the risk of systemic exposure seen with oral terbinafine use. Oral terbinafine is currently the gold standard for treating onychomycosis but associated with safety issues, including drug interactions and liver damage. MOB-015 has been granted marketing authorization in 13 countries. The approval is supported by two Phase 3 trials where MOB-015 demonstrated superior levels of mycological cure (76% vs up to 42% for comparators), and a significantly better complete cure rate compared to vehicle, without any serious adverse reactions. A North American Phase 3 study is ongoing at 33 study centers in the USA and Canada, with a total of 384 patients. The patients are being evaluated over 52 weeks and the primary endpoint will be the proportion of subjects achieving complete cure of their target nail.
About Moberg Pharma, www.mobergpharma.com
Moberg Pharma AB (publ) is a Swedish pharmaceutical company focused on commercializing proprietary innovations based on drug delivery of proven compounds. The company's drug MOB-015, is a novel topical treatment for onychomycosis (nail fungus) with market approval in 13 EU countries. MOB-015 is sold in Sweden under the brand name Terclara® and is available at all pharmacy chains. Phase 3 clinical trials for MOB-015 involving more than 800 patients indicate that the product has the potential to become the future market leader in onychomycosis. Moberg Pharma has agreements with commercial partners in place in various regions including Europe and Canada. Moberg Pharma is headquartered in Stockholm and the company's shares are listed under Small Cap on Nasdaq Stockholm (OMX: MOB).
This information was brought to you by Cision http://news.cision.com
https://news.cision.com/moberg-pharma/r/moberg-pharma-lowers-expectations-on-primary-endpoint-in-ongoing-phase-3-trial-following-data-in-a-s,c4036991
The following files are available for download:
https://mb.cision.com/Main/1662/4036991/2998524.pdf | Moberg Pharma lowers expectations on primary endpoint in ongoing phase 3 trial following data in a subset of patients |
SOURCE Moberg Pharma

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In GET STARTED