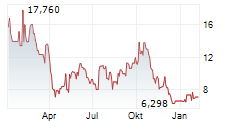
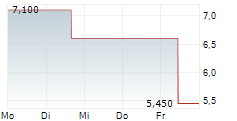
WASHINGTON (dpa-AFX) - Adicet Bio (ACET) reported ADI-001 clinical biomarker data from the Phase 1 GLEAN trial which further reinforces the potential of ADI-001 as a best-in-class allogeneic cell therapy for autoimmune diseases. ADI-001 showed robust tissue trafficking resulting in high levels of ADI-001, significant chimeric antigen receptor T cell activation, and complete CD19+ B cell depletion in secondary lymphoid tissue.
'These results clearly support the potential of ADI-001 and Adicet's off-the-shelf gamma delta CAR T cell platform, by demonstrating robust trafficking and complete B cell depletion in tissue, while providing superior exposure of ADI-001 in secondary lymphoid tissue compared to published third-party data reported for alpha-beta CAR T therapies,' said Blake Aftab, Chief Scientific Officer.
Adicet is advancing the ADI-001 clinical program, and expects to report initial clinical data in the first half of 2025.
For More Such Health News, visit rttnews.com.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News