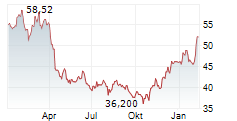
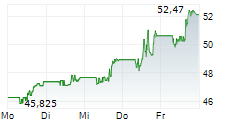
NEW YORK CITY (dpa-AFX) - 2seventy bio (TSVT) announced that the company, in partnership with study sponsor Bristol Myers Squibb, will discontinue enrollment in Phase 3 KarMMa-9 study evaluating Abecma with lenalidomide maintenance versus lenalidomide maintenance alone in patients with newly diagnosed multiple myeloma who have suboptimal response to autologous stem cell transplant. 2seventy bio expects this decision will conserve over $80 million in near-term expenditures and accelerate path to breakeven in 2025.
Anna Truppel-Hartmann, chief medical officer, 2seventy bio, said: 'Since we initiated the Phase 3 KarMMa-9 study in NDMM based on the positive data generated in a similar patient population in the KarMMa-2 cohort 2c study, the NDMM treatment landscape has improved considerably with the increasing use of quadruplet therapy induction, incorporation of more aggressive consolidation therapies, and the ongoing optimization of maintenance therapy regimens. As a result, there are considerably fewer eligible patients than when the study was first designed.'
Also, 2seventy reported continued positive momentum in Abecma's expected return to growth in the earlier line setting following the FDA's approval in April 2024. The company expects third quarter Abecma U.S. revenue growth of approximately 30% from second quarter revenue.
For More Such Health News, visit rttnews.com.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News