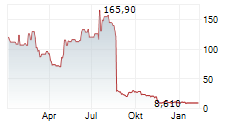
IRVINE, CA / ACCESSWIRE / September 26, 2024 / enVVeno Medical Corporation (NASDAQ:NVNO) ("enVVeno" or the "Company"), a company setting new standards of care for the treatment of venous disease, today announced that it is proposing to offer and sell, subject to market conditions, shares of its common stock (or pre-funded warrants in lieu thereof) in an underwritten public offering. enVVeno expects to grant the underwriter a 30-day option to purchase up to an additional 15% of the number of shares of common stock and pre-funded warrants to be offered in this public offering on the same terms and conditions. The offering is subject to market and other conditions, and there can be no assurance as to whether or when the offering may be completed, or as to the actual size or terms of the offering.
enVVeno intends to use the net proceeds from the offering primarily for the continued development of its two lead products, VenoValve and enVVe, and for general corporate purposes, including working capital.
Titan Partners Group, a division of American Capital Partners, is acting as sole bookrunner for this offering.
The securities described above will be offered pursuant to a shelf registration statement on Form S-3 (File No. 333-273546), which was previously filed with the Securities and Exchange Commission ("SEC") and became effective on August 23, 2023. A preliminary prospectus supplement and accompanying base prospectus relating to and describing the terms of the offering will be filed with the SEC and will be available on the SEC's website located at http://www.sec.gov, copies of which may be obtained, when available, for free by contacting Titan Partners Group LLC, a division of American Capital Partners, LLC, 4 World Trade Center, 29th Floor, New York, New York 10007, by phone at (929) 833-1246 or by email at prospectus@titanpartnersgrp.com.
This offering will be made only by means of a prospectus. This press release shall not constitute an offer to sell or the solicitation of an offer to buy any of the securities described herein, nor shall there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to the registration or qualification under the securities laws of any such state or other jurisdiction.
About enVVeno Medical Corporation
enVVeno Medical Corporation (NASDAQ: NVNO) is an Irvine, California-based, late clinical-stage medical device company focused on the advancement of innovative bioprosthetic (tissue-based) solutions to improve the standard of care for the treatment of venous disease. The Company's lead product, the VenoValve®, is a first-in-class surgical replacement venous valve being developed for the treatment of deep venous Chronic Venous Insufficiency (CVI). The Company is also developing a non-surgical, transcatheter based replacement venous valve for the treatment of deep venous CVI called enVVe®. CVI occurs when valves inside of the veins of the leg become damaged, resulting in the backwards flow of blood (reflux), blood pooling in the lower leg, increased pressure in the veins of the leg (venous hypertension) and in severe cases, venous ulcers that are difficult to heal and become chronic. Both the VenoValve® and enVVe® are designed to act as one-way valves, to help assist in propelling blood up the leg, and back to the heart and lungs. The VenoValve® is currently being evaluated in the SAVVE U.S. pivotal trial and the Company is currently performing the final testing necessary to seek approval for the enVVe® pivotal trial.
Cautionary Note on Forward-Looking Statements
Except for historical information contained herein, this press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, but are not limited to, statements about the Company's expectations regarding the completion, terms, size, and timing of the public offering, and with respect to granting the underwriters a 30-day option to purchase additional shares. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. These statements are based on management's current expectations and beliefs and are subject to a number of risks, uncertainties and assumptions that could cause actual results to differ materially from those described in the forward-looking statements, including risks and uncertainties related to completion of the public offering on the anticipated terms or at all, market conditions and the satisfaction of customary closing conditions related to the public offering, and those other factors described in our risk factors set forth in our filings with the Securities and Exchange Commission from time to time, including our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K. We undertake no obligation to update the forward-looking statements contained herein or to reflect events or circumstances occurring after the date hereof, other than as may be required by applicable law
Investor Contact:
Jenene Thomas, JTC Team, LLC
NVNO@jtcir.com
(908) 824-0775
SOURCE: enVVeno Medical Corporation