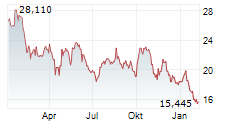
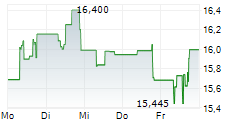
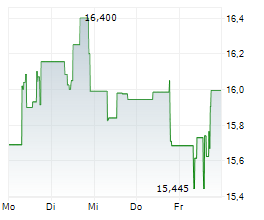
LONDON (dpa-AFX) - Daiichi Sankyo Company Limited (DSKYF.PK) and AstraZeneca's (AZN.L) supplemental Biologics License Application (sBLA) for Enhertu has been granted priority review by the U.S. Food and Drug Administration (FDA) for the treatment of adult patients with HER2 low or HER2 ultralow metastatic breast cancer who have received at least one line of endocrine therapy.
The Prescription Drug User Fee Act (PDUFA) date, or a decision from the regulator is expected on February 1, 2025.
The sBLA is based on data from DESTINY-Breast06 phase 3 study which showed a statistically significant and clinically meaningful progression-free survival benefit for Enhertu.
Enhertu is indicated to treat various cancers including HER2-positive breast cancer, HER2-low breast cancer, HER2-mutant non-small cell lung cancer, HER2-positive gastric or gastroesophageal junction adenocarcinoma, and HER2-positive solid tumors.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News