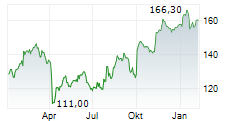
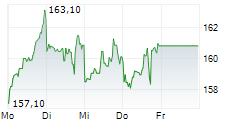
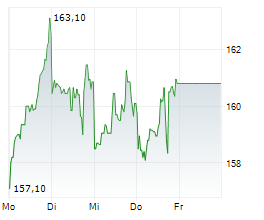
LONDON (dpa-AFX) - AstraZeneca reported positive high-level results from the BATURA Phase IIIb trial, which showed AIRSUPRA met the primary endpoint, demonstrating a statistically significant and clinically meaningful reduction in the risk of a severe exacerbation when used as an as-needed rescue medication in response to symptoms compared to as-needed albuterol. The trial included patients with intermittent or mild persistent asthma. The safety and tolerability of AIRSUPRA in the BATURA trial was consistent with its established profile.
Sharon Barr, Executive Vice-President, BioPharmaceuticals R&D, AstraZeneca, said: 'The impressive BATURA trial results add to the body of evidence supporting AIRSUPRA as a first-in-class rescue treatment and its role in reducing the risk of asthma exacerbations in patients regardless of their disease severity, and reducing the need for systemic corticosteroids.'
For More Such Health News, visit rttnews.com.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News