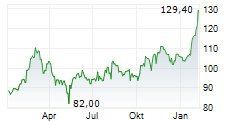
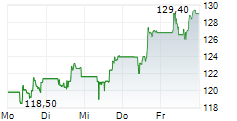
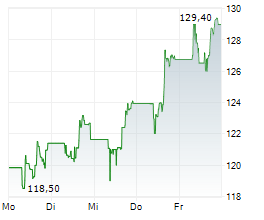
FOSTER CITY (dpa-AFX) - Gilead Sciences Inc. (GILD) announced that additional data from its pivotal Phase 3 PURPOSE 2 trial demonstrated a 96% reduction in HIV infections with Lenacapavir compared to the background HIV incidence among a geographically diverse population of cisgender men and gender-diverse individuals.
The new data are being presented during an oral abstract session on October 8 at the 5th HIV Research for Prevention Conference (HIVR4P) in Lima, Peru.
According to the company, Lenacapavir was highly effective at reducing infections among trial participants: 99.9% of participants did not acquire HIV in the lenacapavir group, with 2 incident cases among 2,179 participants. The results demonstrated superiority of twice-yearly lenacapavir over bHIV, with 96% relative risk reduction, compared with 9 incident cases among 1,086 individuals in the Truvada group. Additionally, twice-yearly lenacapavir was 89% more effective than once-daily Truvada.
Gilead said it will begin regulatory filings for Lenacapavir for pre-exposure prophylaxis (PrEP) by the end of 2024. The company also recently announced voluntary licensing partnerships aimed at expanding future access to Lenacapavir in high-incidence, resource-limited countries.
For More Such Health News, visit rttnews.com.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News