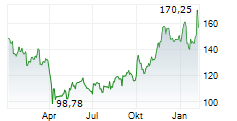
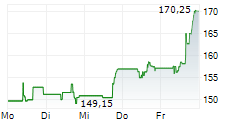
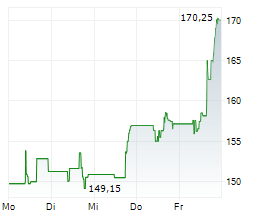
WESTON (dpa-AFX) - Biogen (BIIB) announced that felzartamab has received Breakthrough Therapy Designation from the FDA for the treatment of late antibody-mediated rejection without T-cell mediated rejection in kidney transplant patients. The company noted that the designation provides additional opportunities to engage the FDA and to support the drug development program through Fast Track designation features. Biogen plans to initiate Phase 3 trials for felzartamab across AMR, IgAN, and PMN in 2025.
Felzartamab was originally developed by MorphoSys for multiple myeloma. HI-Bio exclusively licensed the rights to develop and commercialize felzartamab across all indications in all countries and territories excluding China. Biogen acquired HI-Bio in July 2024.
For More Such Health News, visit rttnews.com.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News