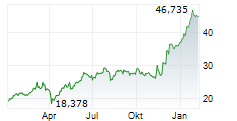
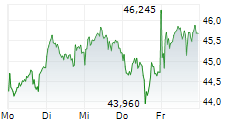
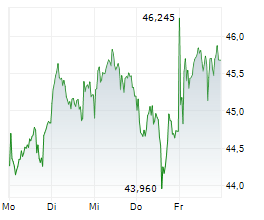
LEVERKUSEN (dpa-AFX) - German pharmaceutical company Bayer AG (BAYZF.PK, BAYRY.PK, BYR.L) announced Wednesday that the U.S. Food and Drug Administration (FDA) has accepted the company's New Drug Application (NDA) for the investigational compound elinzanetant seeking approval for the treatment of moderate-to-severe vasomotor symptoms (VMS, also known as hot flashes) associated with menopause.
If approved, elinzanetant will offer a new non-hormonal option to women seeking treatment for moderate to severe vasomotor symptoms.
The NDA application is based on the positive results from the OASIS 1, 2 and 3 Phase III studies evaluating the efficacy and safety of the investigational compound elinzanetant versus placebo.
Bayer is continuing to submit applications for marketing authorizations of elinzanetant to further health authorities as well.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News