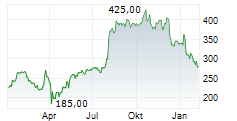
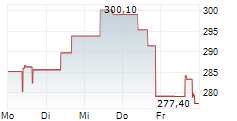
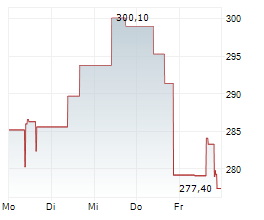
WASHINGTON (dpa-AFX) - RNAi therapeutics company Alnylam Pharmaceuticals, Inc. (ALNY) announced Wednesday the submission of its supplemental New Drug Application (sNDA) to the U.S. Food and Drug Administration (FDA) for vutrisiran, an investigational RNAi therapeutic in development for the treatment of ATTR amyloidosis with cardiomyopathy (ATTR-CM).
Vutrisiran is the generic name for AMVUTTRA, which is currently approved by the U.S. FDA for the treatment of the polyneuropathy of hereditary ATTR amyloidosis in adults.
As part of the submission, the Company utilized a Priority Review Voucher, which obligates the FDA to an accelerated review timeline.
The sNDA application to the FDA was based on positive results from HELIOS-B, a Phase 3, randomized, double-blind, placebo-controlled multicenter global study in patients with ATTR-CM with substantial background use of other effective therapies.
The study demonstrated favorable effects of vutrisiran on outcomes of death and cardiovascular events, functional capacity and quality of life in patients with ATTR-CM.
The safety profile of vutrisiran in HELIOS-B was consistent with the established profile of the drug. In HELIOS-B, rates of adverse events (AEs), serious AEs, severe AEs, and AEs leading to study drug discontinuation were similar between the vutrisiran and placebo arms.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News