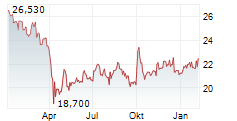
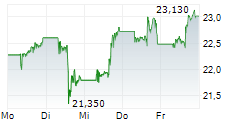
NEW YORK CITY (dpa-AFX) - Pfizer Inc. (PFE) announced Friday that the U.S. Food and Drug Administration has approved HYMPAVZI (marstacimab-hncq) for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adults and pediatric patients 12 years of age and older with hemophilia A (congenital factor VIII deficiency) without factor VIII (FVIII) inhibitors, or hemophilia B (congenital factor IX deficiency) without factor IX (FIX) inhibitors.
The approval is based on Phase 3 study results demonstrating substantial bleed reduction compared to routine prophylaxis and on-demand treatment in eligible patients with hemophilia A or B without inhibitors.
In the U.S., HYMPAVZI is the first once-weekly subcutaneous prophylactic treatment for eligible individuals living with hemophilia B, and it is also the first to be administered via a pre-filled pen or syringe for those living with either hemophilia A or B.
Hemophilia is a family of rare genetic blood diseases caused by a clotting factor deficiency (FVIII in hemophilia A, FIX in hemophilia B), impacting more than 800,000 people globally.
Diagnosed in early childhood, hemophilia inhibits the blood's ability to clot properly, increasing the risk of repeated bleeding inside the joints, which can lead to permanent joint damage.
For More Such Health News, visit rttnews.com.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News