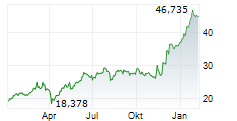
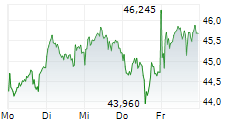
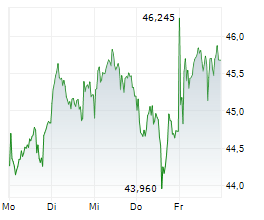
LEVERKUSEN (dpa-AFX) - Bayer announced the submission of an application to the European Medicines Agency for the oral androgen receptor inhibitor darolutamide. The company is seeking approval for the use of darolutamide in combination with androgen deprivation therapy in patients with metastatic hormone-sensitive prostate cancer.
Darolutamide is developed jointly by Bayer and Orion, a Finnish pharmaceutical company. The submission is based on positive results from the pivotal Phase III ARANOTE trial. The compound is already approved in metastatic hormone-sensitive prostate cancer, under the brand name Nubeqa, in combination with androgen deprivation therapy and docetaxel in over 80 markets around the world.
For More Such Health News, visit rttnews.com.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News