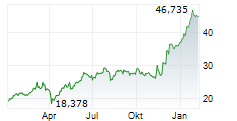
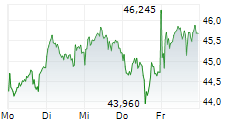
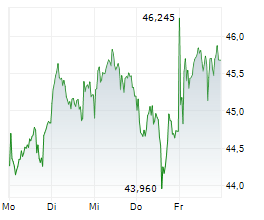
LEVERKUSEN (dpa-AFX) - Bayer AG (BAYZF.PK, BAYRY.PK, BYR.L) Tuesday announced that it has submitted marketing authorization application to the European Medicines Agency for Elinzanetant to treat vasomotor symptoms or hot flashes in women.
Elinzanetant is a dual neurokinin-1 and 3 receptor antagonist, in late-stage clinical development for the non-hormonal treatment of moderate to severe vasomotor symptoms. The EMA submission is based on results from the Phase III development program OASIS.
Hot flashes are due to a decrease of estrogen, which can result from the progressive reduction of ovarian function due to natural menopause or medical intervention.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News