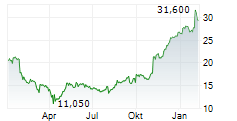
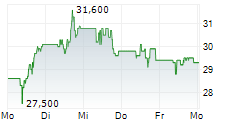
PETAH TIKVA (dpa-AFX) - Amicus Therapeutics (FOLD) announced a License Agreement with Teva Pharmaceuticals USA, Inc. and Teva Pharmaceuticals Inc. resolving the patent litigation initiated by Amicus in response to Teva's Abbreviated New Drug Application (ANDA) for a generic version of GALAFOLD (migalastat) 123 mg capsules, aimed at obtaining approval before the relevant patents expire.
As per the terms of the Agreement, Amicus will grant Teva a license to market its generic version of GALAFOLD in the United States beginning on January 30, 2037, if approved by the U.S. Food and Drug Administration (FDA) and unless certain limited circumstances customarily included in these types of agreements occur.
The parties will terminate all ongoing Hatch-Waxman litigation between Amicus and Teva regarding GALAFOLD patents pending in the U.S. District Court for the District of Delaware. The litigation will continue against Aurobindo1 as the remaining active party and the litigation stay remains in place for Lupin.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News