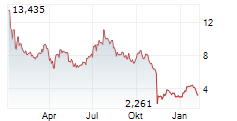
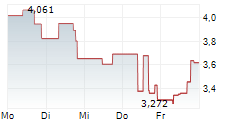
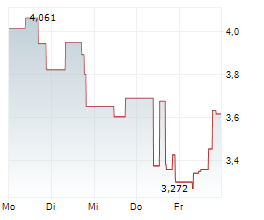
WASHINGTON (dpa-AFX) - Anavex Life Sciences Corp. (AVXL) announced promising preliminary electroencephalography or EEG biomarker results from Part A of its ongoing placebo-controlled Phase 2 clinical study of ANAVEX 3-71 for the treatment of schizophrenia.
Part A of the Phase 2 study ANAVEX 3-71-SZ-001, was a multiple ascending dose study in 16 participants treated with either oral placebo, oral ANAVEX3-71 90 mg daily, or oral ANAVEX3-71 180 mg daily for 10 days.
According to the company, preliminary results demonstrated a dose-dependent effect of ANAVEX 3-71 on two key EEG biomarkers in patients with schizophrenia. Treatment with ANAVEX3-71 compared to placebo resulted in improvements in 40 Hz Auditory Steady-State Response (ASSR) Inter Trial Coherence (ITC) and Resting State Alpha Power, both of which were increased. The effects were most pronounced in the higher dose group demonstrating a dose-dependent pharmacodynamic effect.
Anavex noted that the results provide evidence of central nervous system (CNS) target engagement and potential therapeutic effects of ANAVEX3-71 in schizophrenia.
The company said that ANAVEX3-71 was well tolerated, with no serious adverse events reported.
The currently ongoing Part B of the placebo-controlled Phase 2 study, which includes more participants and a longer treatment duration, will provide more comprehensive data on the efficacy and safety of ANAVEX3-71 in schizophrenia, the company said.
Anavex expects data from Part B of the placebo-controlled Phase 2 study in the first half of 2025.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News