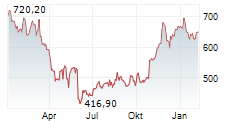
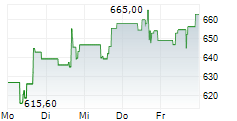
WASHINGTON (dpa-AFX) - Regeneron Pharmaceuticals Inc. (REGN) announced positive three-year (156-week) data for EYLEA HD (aflibercept) Injection 8 mg from an extension study of the Phase 3 PHOTON trial in patients with diabetic macular edema (DME).
At three years, the longer-term data showed the vast majority of EYLEA HD patients who entered the extension study sustained the visual gains and anatomic improvements achieved by the end of the second year, while achieving substantially longer treatment intervals than have been previously demonstrated.
Notably, patients switched to EYLEA HD experienced substantially slower fluid reaccumulation following their first EYLEA HD dose. The achievement of much longer dosing intervals with EYLEA HD - together with the notably slower fluid reaccumulation - supports the longer duration of action of EYLEA HD.
At week 156 of the extension study of the Phase 3 PHOTON trial, 88% of EYLEA HD patients had a last assigned dosing interval of 12 weeks or more, while maintaining the visual and anatomic improvements achieved in the first 96 weeks.
For More Such Health News, visit rttnews.com.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News