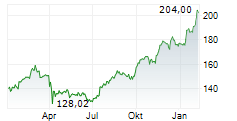
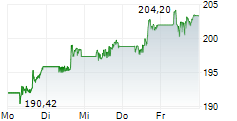
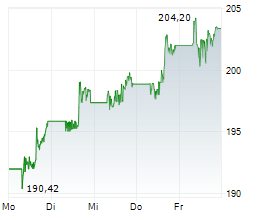
NEW BRUNSWICK (dpa-AFX) - Johnson & Johnson (JNJ) Friday announced positive results from Phase 3b SPECTREM study of Tremfya to treat adults with low body surface area (BSA) moderate plaque psoriasis (PsO) in sensitive or highly visible areas. The study achieved its primary goal.
Data from the study showed that treatment with Tremfya resulted in clear or almost clear skin in majority of adults with low BSA moderate PsO in areas including the scalp, face, skin folds and genitals, who had failed previous topical treatment.
These data were presented at the 2024 Fall Clinical Dermatology Conference.
'People who have special site plaque psoriasis with lesions that cover a smaller total area of their body are often only prescribed topical treatments and not considered candidates for advanced therapies, as treatment decisions are often driven by body surface area coverage and not symptomatic burden,' said Linda Stein Gold, Director of Dermatology Clinical Research at Henry Ford Health, and SPECTREM investigator. 'Results of the SPECTREM study could represent a new approach to care for patients with low body surface area psoriasis, as the majority of patients treated with TREMFYA achieved clear or almost clear skin.'
Tremfya is approved for the treatment of adults with moderate to severe plaque psoriasis who may benefit from systemic therapy or phototherapy.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News