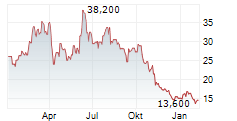
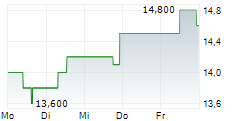
BEIJING (dpa-AFX) - Zai Lab Limited (ZLAB) Tuesday said the Phase 3 study of KarXT for the Treatment of Schizophrenia in China met its primary goal.
In the study, KarXT showed a statistically significant 9.2-point reduction in Positive and Negative Syndrome Scale (PANSS) total score, a measure of schizophrenia symptom severity, at Week 5 compared to placebo.
Further, no new safety signals were found compared to prior KarXT trials in schizophrenia.
Based on these results, the company plans to submit a new drug application to China's National Medical Products Administration (NMPA) in early 2025.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News