Teva Pharmaceutical Industries Ltd. recently unveiled encouraging results from clinical trials, potentially bolstering its position in the competitive pharmaceutical sector. The SOLARIS Phase 3 study for TEV-'749, a subcutaneous long-acting olanzapine injection, demonstrated significant improvements in social functioning and quality of life for adult schizophrenia patients. Notably, no cases of post-injection delirium/sedation syndrome (PDSS) were reported, suggesting a possible safety advantage over existing treatments. Additionally, real-world analyses of UZEDY, an approved long-acting risperidone injection, showed high adherence rates among adults with schizophrenia, particularly those facing treatment barriers.
European Commission Imposes Substantial Fine
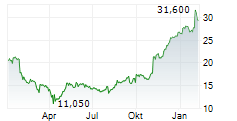
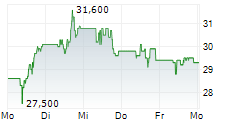
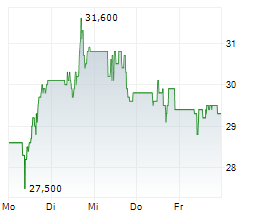
However, Teva faces a considerable setback as the European Commission levied a €462.6 million fine against the company. The pharmaceutical giant is accused of abusing its dominant market position to artificially extend patent protection for its multiple sclerosis drug Copaxone and disseminating misleading information about a competitor's product. Teva has vehemently denied these allegations, deeming them "extreme" and "factually unfounded," and plans to appeal the decision. This development has negatively impacted Teva's stock, which experienced a decline in trading on the New York Stock Exchange.
Ad
Teva Pharmaceutical Stock: New Analysis - 02 NovemberFresh Teva Pharmaceutical information released. What's the impact for investors? Our latest independent report examines recent figures and market trends.
Read our updated Teva Pharmaceutical analysis...