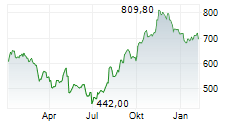
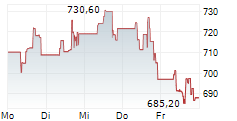
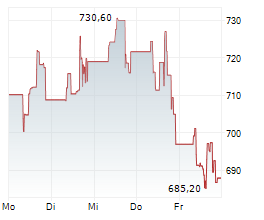
BEIJING (dpa-AFX) - Zai Lab Limited (ZLAB) and argenx SE (ARGX) Monday said China's National Medical Products Administration (NMPA) has approved VYVGART Hytrulo for the treatment of adults with chronic inflammatory demyelinating polyneuropathy (CIDP), a rare type of autoimmune disorder.
VYVGART Hytrulo is the first and only therapy approved in China for the treatment of CIDP.
The NMPA approval is supported by positive results from the ADHERE study evaluating VYVGART Hytrulo in CIDP.
The drug is already approved by the U.S. Food and Drug Administration (FDA) for the treatment of CIDP and generalized myasthenia gravis (gMG).
Zai Lab has acquired rights from argenx to develop and sell VYVGART Hytrulo in mainland China, Hong Kong, Macau, and Taiwan.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News