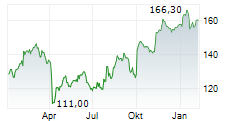
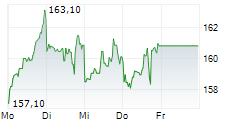
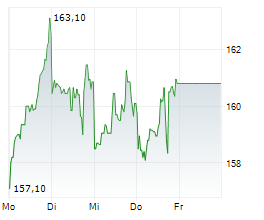
LONDON (dpa-AFX) - AstraZeneca PLC (AZN.L) and Daiichi Sankyo Company, Limited have submitted a new Biologics License Application (BLA) for accelerated approval of datopotamab deruxtecan in the U.S. for the treatment of patients with previously treated epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC).
The new BLA is based on results from TROPION-Lung05 Phase II study and supported by data from the TROPION-Lung01 Phase III and TROPION-PanTumor01 Phase I studies. The companies have withdrawn the previously submitted BLA based on TROPION-Lung01 Phase III study for patients with nonsquamous NSCLC.
AstraZeneca and Daiichi Sankyo are evaluating datopotamab deruxtecan alone and with Tagrisso in EGFR-mutated nonsquamous NSCLC in the ongoing TROPION-Lung14 and TROPION-Lung15 Phase III trials. Phase III trials dubbed AVANZAR and TROPION-Lung10 in 1st-line advanced or metastatic nonsquamous NSCLC are also underway. The companies also plan an additional trial in patients with biomarker-positive tumours in the 2nd-line nonsquamous NSCLC setting.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News