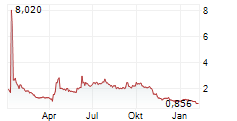
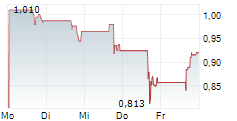
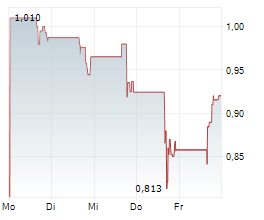
Marlborough, Massachusetts--(Newsfile Corp. - November 14, 2024) - Phio Pharmaceuticals Corp. (NASDAQ: PHIO) is a clinical-stage biotechnology company exploring new pathways towards a cancer-free future by using its INTASYL® siRNA gene silencing technology designed to make the body's immune cells more effective in killing cancer cells today reported its financial results for the quarter ended September 30, 2024 and provided a business update.
Recent Corporate Updates
The Phase 1b dose escalation clinical trial for our lead product candidate, PH-762, previously received a positive safety recommendation to advance to the next highest dose from the Safety Monitoring Committee (SMC) for the first cohort. The second cohort is fully enrolled, and screening for the 3rd cohort will open on 10 Dec 2024. Each enrolled subject receives four (4) intratumoral (IT) doses of PH-762. The currently enrolled patients were diagnosed with cutaneous squamous cell carcinoma (6 patients) and metastatic melanoma (1 patient). Two of the three patients in the second cohort have completed the treatment and the follow-up phase.
At Day 36 (tumor excision), while each patient in the first cohort had stable disease, the following results were described for two patients who have completed treatment in the second cohort:
Complete response (100% tumor clearance) reported for 1 patient with cutaneous squamous cell carcinoma
Partial response (90% tumor clearance) reported for 1 patient with cutaneous squamous cell carcinoma
A sixth clinical trial site, Paradigm Clinical Research Centers in San Diego, CA, was selected for the Phase 1b study of PH-762 and will join the other five sites engaged in the study: Banner MD Anderson Cancer Center in Gilbert, Arizona. The George Washington University-Medical Faculty Associates in Washington, D.C; The University of Pittsburgh Medical Center (UPMC) Department of Dermatology; Integrity Research Clinical Associates in Delray Beach, Florida; and Centricity Research in Dublin Ohio.
Cancer Immunology, Immunotherapy, a peer reviewed journal, highlighted the INTASYL compound PH-804. The article, issued on Oct 3, 2024, was authored by M Maxwell et al. entitled "INTASYL Self-Delivering RNAi Decreases TIGIT Expression, Enhancing NK Cell Cytotoxicity: A Potential Application to Increase the Efficacy of NK-Adoptive Cell Therapy Against Cancer".
Presented new data on Phio's INTASYL self-delivering siRNA technology applications for immuno-oncology therapy at several conferences:
9th Annual CAR-TCR Summit held on Sept 17th showcased INTASYL siRNA technology-an innovative and flexible solution designed specifically to enhance cell potency and improve overall yield for adoptive cell therapy.
20th Annual Oligonucleotide Therapeutics Society (OTS) held on October 8th showcased INTASYL's role in helping immune cells target and kill cancer cells.
The American Society of Gene and Cell Therapy's 2024 Advancing Cancer Conference held Oct 16-17 reported on INTASYL Compound PH-894: A potent and specific silencing of BRD4 in NK Cells.
39th Annual meeting of the Society for Immunotherapy of Cancer (SITC) held November 9th reported on new clinical data from the Phase 1b clinical trial for its INTASYL Compound PH-762.
Phio Pharmaceuticals is currently participating in the Renmark Financial Virtual Non-Deal Roadshow series that features Phio's CEO and Chairman of the Board, Robert Bitterman, presentation on its INTASYL technology. The presentations are directed to the retail investor communities including those in the Chicago, Denver, Dallas, Atlanta, and Boston areas. These roadshows have been on-going since late August 2024.
Financial Results
Cash Position
At September 30, 2024, the Company had cash of $5.4 million as compared with $8.5 million at December 31, 2023.
In July 2024, the Company entered into inducement letter agreements with certain holders of the Company's existing warrants to purchase up to an aggregate of 545,286 shares of common stock at a reduced exercise price of $5.45 per share. In consideration for the immediate exercise of the existing warrants, the Company agreed to issue five and one-half year term Series C warrants to purchase up to 583,098 shares of common stock and eighteen month term Series D warrants to purchase up to 507,474 shares of common stock, both at an exercise price of $5.45. The net proceeds to the Company were approximately $2.6 million, after deducting placement agent fees and offering expenses.
Research and Development Expenses
Research and development expenses were $0.6 million for the three months ended September 30, 2024 as compared with $1.8 million for the three months ended September 30, 2023, a decrease of 64%. The decrease was primarily driven by non-recurring clinal program direct start-up costs in the 3rd Quarter 2023 and reductions in research compensation attributable to reductions in headcount of participants during the 3rd Quarter 2024.
General and Administrative Expenses
General and administrative expenses were $0.95 million for the three months ended September 30, 2024 as compared with $0.97 million for the three months ended September 30, 2023, a decrease of 2%. The decrease was primarily due to decreases in salary-related expenses compared to the prior year period.
Net Loss
Net loss was $1.5 million for the three months ended September 30, 2024 as compared with $2.8 million for the three months ended September 30, 2023. The decrease in net loss was primarily due to the changes in research and development expenses, as described above.
About Phio Pharmaceuticals Corp.
Phio Pharmaceuticals Corp. (NASDAQ: PHIO) is a clinical-stage biotechnology company and a pioneer in the RNAi revolution advancing its proprietary INTASYL siRNA gene silencing technology to eliminate cancer. INTASYL can target and silence virtually any gene with high degree of specificity across a wide range of cell types and tissues. INTASYL is designed to enhance the ability of immune cells to more effectively kill tumor cells. INTASYL has also demonstrated enhancement adoptive cell therapy. Notably, INTASYL is a self-delivering RNAi technology focused on immuno-oncology therapeutics without the need for formulation enhancements or manipulations to reach its target.
Phio's lead clinical program, PH-762, is an INTASYL compound that silences PD-1. PH-762 is a potential non-surgical treatment for skin cancers. The on-going Phase 1b trial (NCT# 06014086) received FDA clearance for an Investigational New Drug Application to evaluate PH-762 in the treatment of cutaneous SCC, melanoma and Merkel cell carcinoma in second quarter of 2023.
For additional information, visit the Company's website, www.phiopharma.com.
Forward Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by words such as "intends," "believes," "anticipates," "indicates," "plans," "expects," "suggests," "may," "would," "should," "potential," "designed to," "will," "ongoing," "estimate," "forecast," "target," "predict," "could" and similar references, although not all forward-looking statements contain these words. Examples of forward-looking statements contained in this press release include, among others, the possibility that our INTASYL® siRNA gene silencing technology will make the body's immune cells more effective in killing cancer cells and statements regarding our commercial and clinical strategy, development plans and timelines and other future events.
These statements are based only on our current beliefs, expectations and assumptions and are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results may differ materially from those indicated in the forward-looking statements as a result of a number of important factors, including, but not limited to, the impact to our business and operations by inflationary pressures, rising interest rates, recession fears, the development of our product candidates, results from our preclinical and clinical activities, our ability to execute on business strategies, our ability to develop our product candidates with collaboration partners, and the success of any such collaborations, the timeline and duration for advancing our product candidates into clinical development, the timing or likelihood of regulatory filings and approvals, the success of our efforts to commercialize our product candidates if approved, our ability to manufacture and supply our product candidates for clinical activities, and for commercial use if approved, the scope of protection we are able to establish and maintain for intellectual property rights covering our technology platform, our ability to obtain future financing, market and other conditions and those identified in our Annual Report on Form 10-K and subsequent Quarterly Reports on Form 10-Q under the caption "Risk Factors" and in other filings the Company periodically makes with the SEC. Readers are urged to review these risk factors and to not act in reliance on any forward-looking statements, as actual results may differ from those contemplated by our forward-looking statements. Phio does not undertake to update forward-looking statements to reflect a change in its views, events or circumstances that occur after the date of this release, except as required by law.
Contact:
Phio Pharmaceuticals Corp.
Jennifer Phillips: jphillips@phiopharma.com
Corporate Affairs
PHIO PHARMACEUTICALS CORP.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(Amounts in thousands, except share and per share data)
(Unaudited)
| Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||
| 2024 | 2023 | 2024 | 2023 | |||||||||||||
| Operating expenses: | ||||||||||||||||
| Research and development | $ | 644 | $ | 1,808 | $ | 2,658 | $ | 5,325 | ||||||||
| General and administrative | 946 | 968 | 3,055 | 3,600 | ||||||||||||
| Total operating expenses | 1,590 | 2,776 | 5,713 | 8,925 | ||||||||||||
| Operating loss | (1,590 | ) | (2,776 | ) | (5,713 | ) | (8,925 | ) | ||||||||
| Total other income (expense), net | 66 | (4 | ) | 189 | (6 | ) | ||||||||||
| Net loss | $ | (1,524 | ) | $ | (2,780 | ) | $ | (5,524 | ) | $ | (8,931 | ) | ||||
| Net loss per common share: | ||||||||||||||||
| Basic and diluted | $ | (1.54 | ) | $ | (10.25 | ) | $ | (8.23 | ) | $ | (45.28 | ) | ||||
| Weighted average number of common shares outstanding | ||||||||||||||||
| Basic and diluted | 990,033 | 271,129 | 670,875 | 197,227 | ||||||||||||
PHIO PHARMACEUTICALS CORP.
CONDENSED CONSOLIDATED BALANCE SHEETS
(Amounts in thousands, except share and per share data)
(Unaudited)
| September 30, 2024 | December 31, 2023 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 5,390 | $ | 8,490 | ||||
| Prepaid expenses and other current assets | 474 | 832 | ||||||
| Total current assets | 5,864 | 9,322 | ||||||
| Right of use asset | - | 33 | ||||||
| Property and equipment, net | 1 | 6 | ||||||
| Other assets | - | 3 | ||||||
| Total assets | $ | 5,865 | $ | 9,364 | ||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 184 | $ | 657 | ||||
| Accrued expenses | 735 | 942 | ||||||
| Lease liability | - | 35 | ||||||
| Total current liabilities | 919 | 1,634 | ||||||
| Total stockholders' equity | 4,946 | 7,730 | ||||||
| Total liabilities and stockholders' equity | $ | 5,865 | $ | 9,364 | ||||
To view the source version of this press release, please visit https://www.newsfilecorp.com/release/230052
SOURCE: Phio Pharmaceuticals Corp.