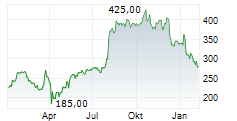
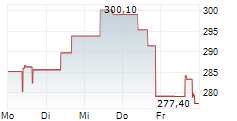
WASHINGTON (dpa-AFX) - Alnylam Pharmaceuticals Inc. (ALNY) has shared new findings from its Phase 1 study of nucresiran (previously known as ALN-TTRsc04), a next-generation RNAi therapy being developed for the treatment of transthyretin (ATTR). The data were presented in an oral session at the American Heart Association Scientific Sessions 2024 in Chicago.
The new results demonstrated that a single dose of nucresiran at 300 mg or higher led to rapid knockdown of serum TTR with low inter-patient variability, with mean reductions of greater than 90% from baseline achieved at Day 15 and sustained through at least Day 180. At these doses, peak reduction of mean TTR levels of greater than 96% were achieved by Day 29.
Furthermore, serum TTR levels remained substantially reduced at Day 360 with a mean reduction of greater than 70% after a single 300 mg dose. Day 360 results are not yet available for the 600 mg and 900 mg dose cohorts. All doses of nucresiran have been well tolerated to date.
The ongoing Phase 1 dose-finding study evaluated the safety, as well as pharmacodynamics and pharmacokinetics, of single doses of nucresiran in healthy subjects.
For More Such Health News, visit rttnews.com.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News