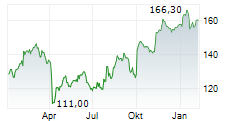
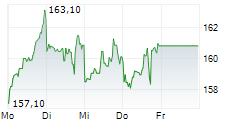
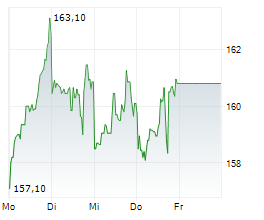
LONDON (dpa-AFX) - AstraZeneca PLC (AZN.L) Friday said that the U.S. Food and Drug Administration or FDA has granted priority review to the company's supplemental Biologics License Application (sBLA) for Imfinzi for the treatment of patients with muscle-invasive bladder cancer.
The Prescription Drug User Fee Act date or a decision form the regulator is expected during the second quarter of 2025.
The sBLA was based on results from the NIAGARA Phase III study, in which perioperative Imfinzi showed a statistically significant and clinically meaningful overall survival benefit.
Based on the NIAGARA results, the company has submitted applications to regulators in the EU, Japan and several other countries, which are under review.
Copyright(c) 2024 RTTNews.com. All Rights Reserved
Copyright RTT News/dpa-AFX
© 2024 AFX News